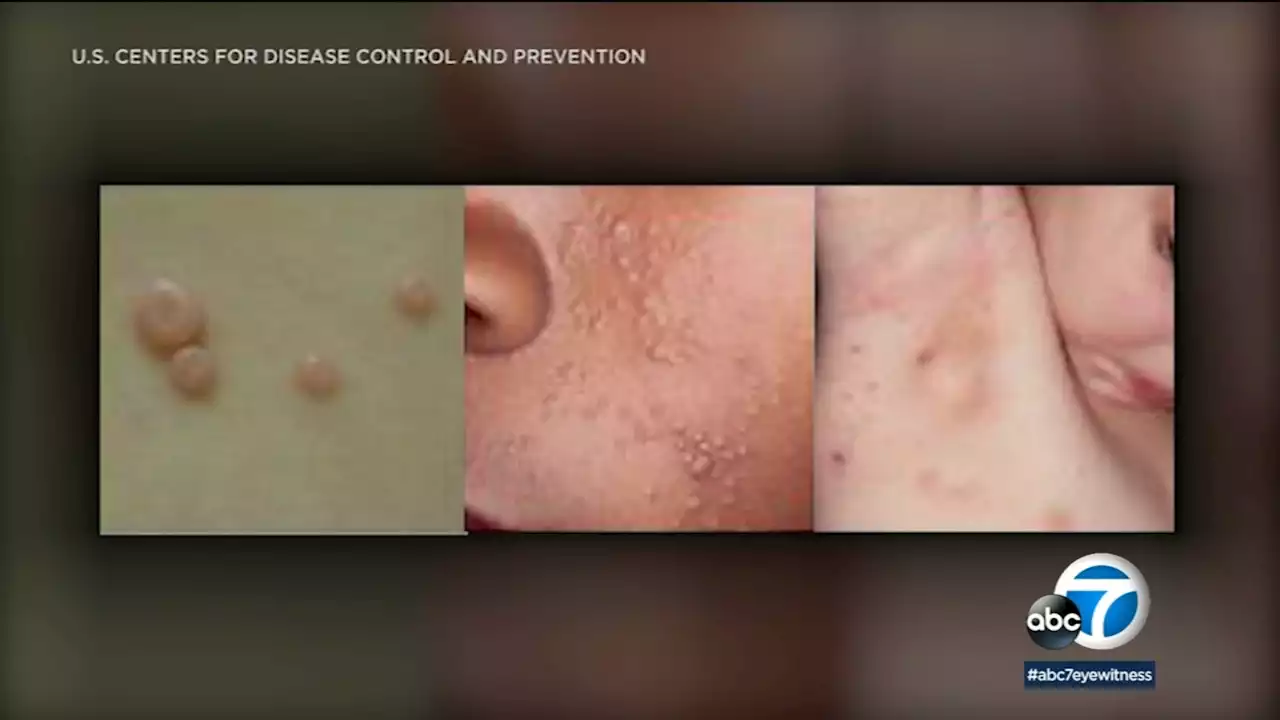
The FDA is warning against self-diagnosis and self-treatment for a common skin condition - a viral infection often called 'water warts.'
The Food and Drug Administration is warning against self-diagnosis and self-treatment for a common skin condition - a viral infection often called "water warts."
One mother from Tustin said they went to three different doctors before they figured out what was wrong with her son. She said her son's red, prickly bumps stuck around for several months until they met dermatologist Dr. Tanya Nino at Providence St. Joseph Hospital in Orange. She figured out it was molluscum.The raised, swollen lesions can appear on any part of the body. Nino said molluscum is a common viral infection spread through skin-to-skin contact.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 To ease cancer drug shortage, FDA will allow imports from ChinaThe FDA is working with a Chinese drugmaker to import the chemotherapy drug cisplatin, a strategy aimed at ramping up supply amid an ongoing shortage of the drug in the U.S.
To ease cancer drug shortage, FDA will allow imports from ChinaThe FDA is working with a Chinese drugmaker to import the chemotherapy drug cisplatin, a strategy aimed at ramping up supply amid an ongoing shortage of the drug in the U.S.
Leer más »
 The Garage Food Hall nominated for USA Today's Best Food HallVoting ends Monday, July 3 and the winners will be announced on Friday, July 14. You can vote once a day, here.
The Garage Food Hall nominated for USA Today's Best Food HallVoting ends Monday, July 3 and the winners will be announced on Friday, July 14. You can vote once a day, here.
Leer más »
 FDA clears Lynparza for prostate cancer treatmentThe Food and Drug Administration cleared a new cancer-fighting drug called Lynparza for prostate cancer treatment.
FDA clears Lynparza for prostate cancer treatmentThe Food and Drug Administration cleared a new cancer-fighting drug called Lynparza for prostate cancer treatment.
Leer más »
 People are confusing ‘poppers’ for energy shots — and dying, FDA warns“Make no mistake, ingesting or inhaling poppers seriously jeopardizes your health,” said a pharmacist who works for the FDA.
People are confusing ‘poppers’ for energy shots — and dying, FDA warns“Make no mistake, ingesting or inhaling poppers seriously jeopardizes your health,” said a pharmacist who works for the FDA.
Leer más »
 FDA OKs Injectafer for Iron Deficiency Anemia in HFThis new indication for ferric carboxymaltose injection marks the first FDA approval of an intravenous iron replacement therapy for adult patients with heart failure, the company said.
FDA OKs Injectafer for Iron Deficiency Anemia in HFThis new indication for ferric carboxymaltose injection marks the first FDA approval of an intravenous iron replacement therapy for adult patients with heart failure, the company said.
Leer más »
 FDA OKs Letermovir to Prevent CMV in Kidney TransplantsLetermovir was shown to be just as effective as valganciclovir in preventing CMV in kidney transplant recipients in new study published in JAMA; results prompt additional FDA approval for this indication.
FDA OKs Letermovir to Prevent CMV in Kidney TransplantsLetermovir was shown to be just as effective as valganciclovir in preventing CMV in kidney transplant recipients in new study published in JAMA; results prompt additional FDA approval for this indication.
Leer más »