The CDC signed off on certain young children receiving an updated Covid-19 booster, clearing the way for the shots to be widely available at pediatricians’ offices and other vaccination sites
Physicians are reporting high numbers of respiratory illnesses like RSV and the flu earlier than the typical winter peak. WSJ’s Brianna Abbott explains what the early surge means for the coming winter months. Photo illustration: Kaitlyn WangThe Centers for Disease Control and Prevention signed off on certain young children receiving an, clearing the way for the shots to be widely available at pediatricians’ offices and other vaccination sites.
The agency’s move on Friday was the last step before children as young as 6 months could get the shots, which were modified to target offshoots of the Omicron variant as well as the original strain of the virus.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
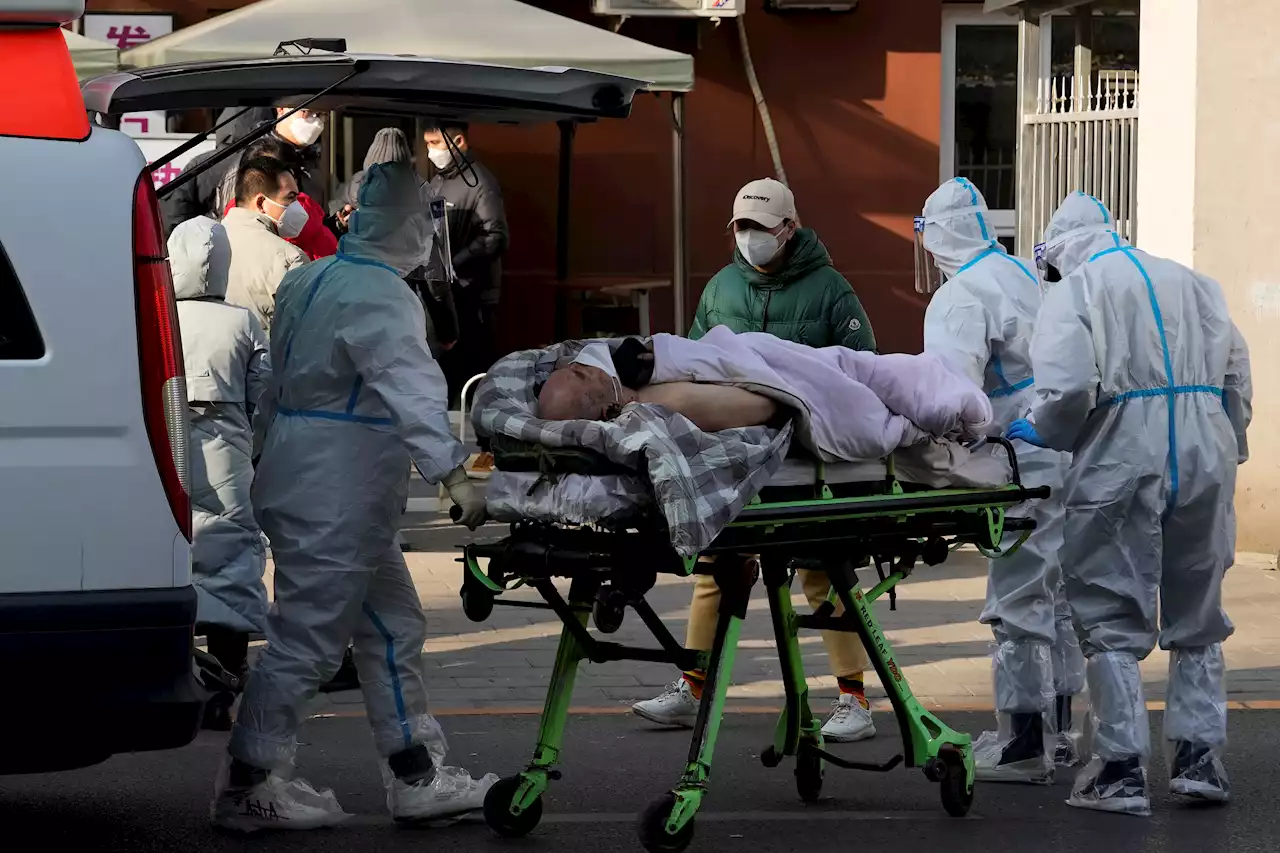 China Struggles With COVID Wave After 'Zero-COVID' EasesA rash of COVID-19 cases in schools and businesses were reported by social media users Friday in areas across China after the ruling Communist Party loosened anti-virus rules as it tries to reverse a deepening economic slump.
China Struggles With COVID Wave After 'Zero-COVID' EasesA rash of COVID-19 cases in schools and businesses were reported by social media users Friday in areas across China after the ruling Communist Party loosened anti-virus rules as it tries to reverse a deepening economic slump.
Leer más »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Leer más »
 Some children as young as 6 months now eligible for COVID-19 booster shotsAlthough the updated boosters have been available since September, only a small percentage of Americans have gotten one.
Some children as young as 6 months now eligible for COVID-19 booster shotsAlthough the updated boosters have been available since September, only a small percentage of Americans have gotten one.
Leer más »
 U.S. FDA authorizes bivalent COVID shots for kids as young as 6 months oldThe amended authorization on Thursday from the Food and Drug Administration allows use of Moderna's bivalent shot as a booster in children 6 months through 5 years of age, two months after their initial vaccination. Pfizer/BioNTech's updated shot can now be given as a third dose to those aged 6 months through 4 years, who have not completed their primary vaccination series or are yet to receive the third dose. Children who have completed their initial three-dose vaccination with Pfizer's original shot are not yet eligible to receive the bivalent booster, the agency said.
U.S. FDA authorizes bivalent COVID shots for kids as young as 6 months oldThe amended authorization on Thursday from the Food and Drug Administration allows use of Moderna's bivalent shot as a booster in children 6 months through 5 years of age, two months after their initial vaccination. Pfizer/BioNTech's updated shot can now be given as a third dose to those aged 6 months through 4 years, who have not completed their primary vaccination series or are yet to receive the third dose. Children who have completed their initial three-dose vaccination with Pfizer's original shot are not yet eligible to receive the bivalent booster, the agency said.
Leer más »
