The amended authorizations allow use of Moderna's bivalent shot in children 6 months through 5 years of age, while the Pfizer/BioNTech vaccine can be given to those aged 6 months through 4 years.
-The U.S. health regulator has authorized COVID-19 shots from Moderna and Pfizer and its partner BioNTech that target both the original coronavirus and Omicron sub-variants for use in children as young as 6 months of age.
Pfizer/BioNTech's updated shot can now be given as a third dose to those aged 6 months through 4 years, who have not completed their primary vaccination series or are yet to receive the third dose. The regulator added that data supporting use of Pfizer/BioNTech's bivalent shot as a booster in this age group is expected in January.
Government data shows that only 2.7% children under the age of two and less than 5% of children aged two to four years who are eligible have completed their primary vaccination series as of Nov. 30, representing a slow uptake of the initial vaccine doses in young children.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
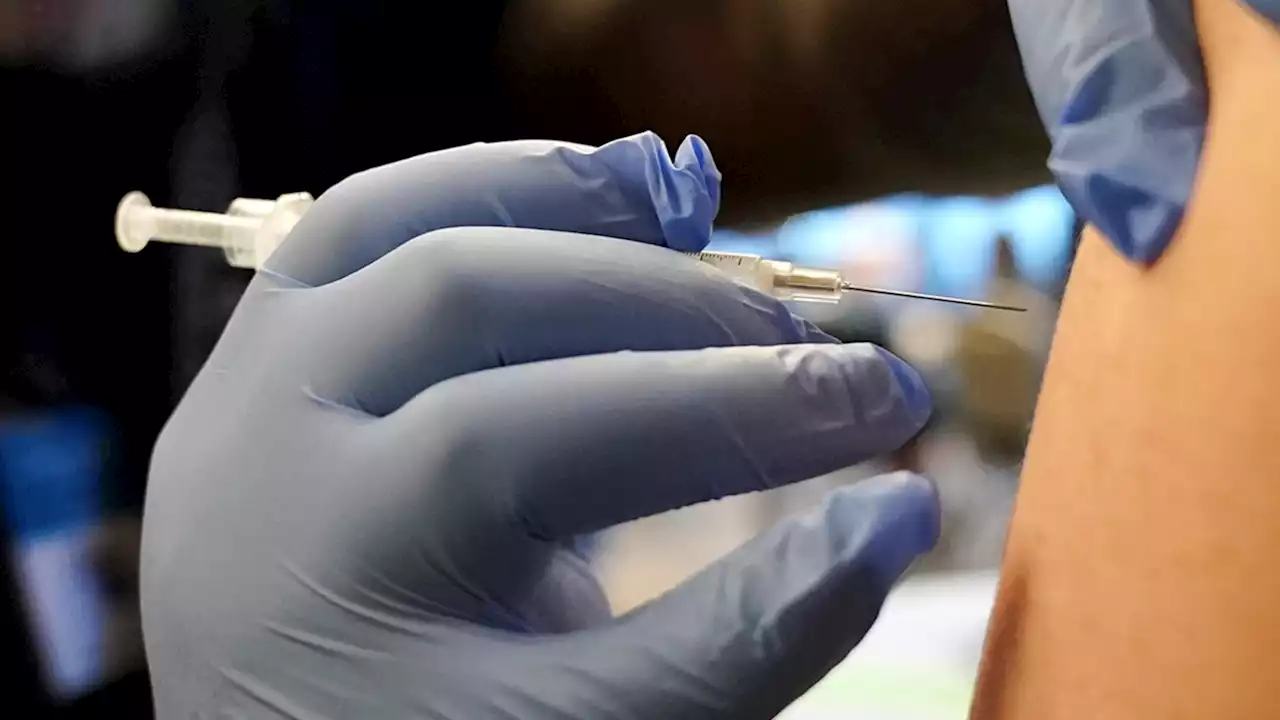 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Leer más »
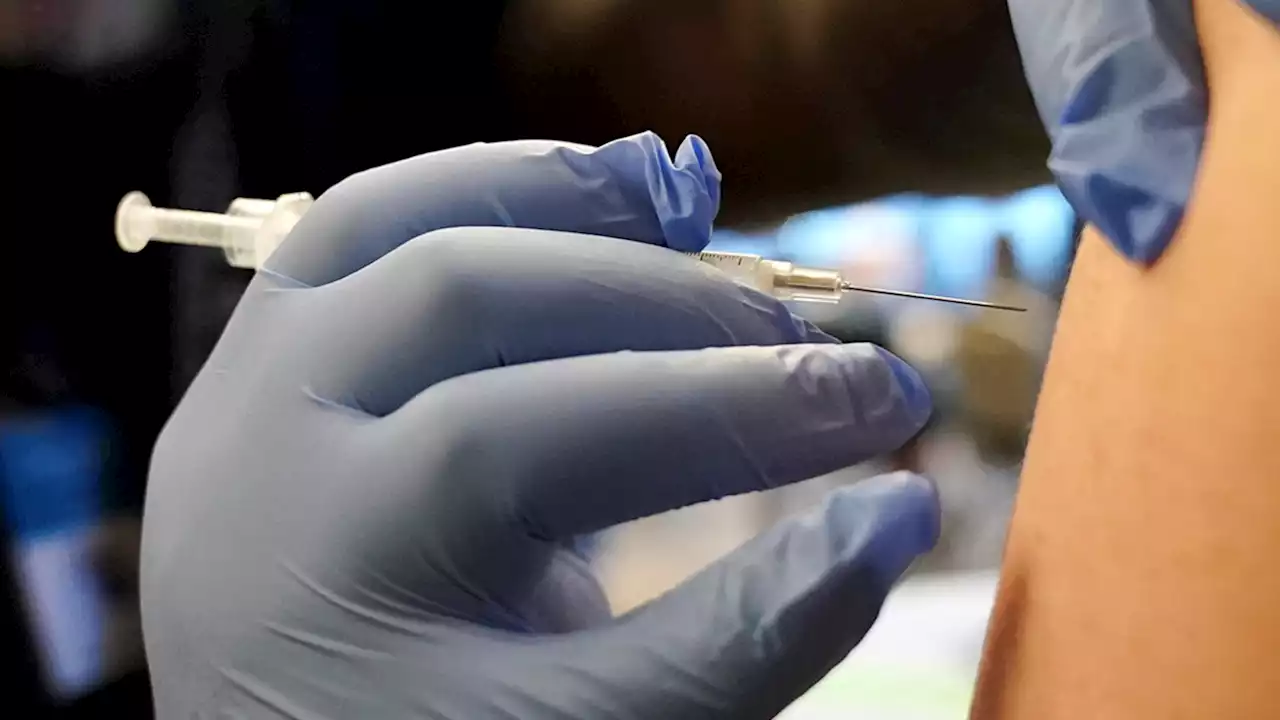 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Leer más »
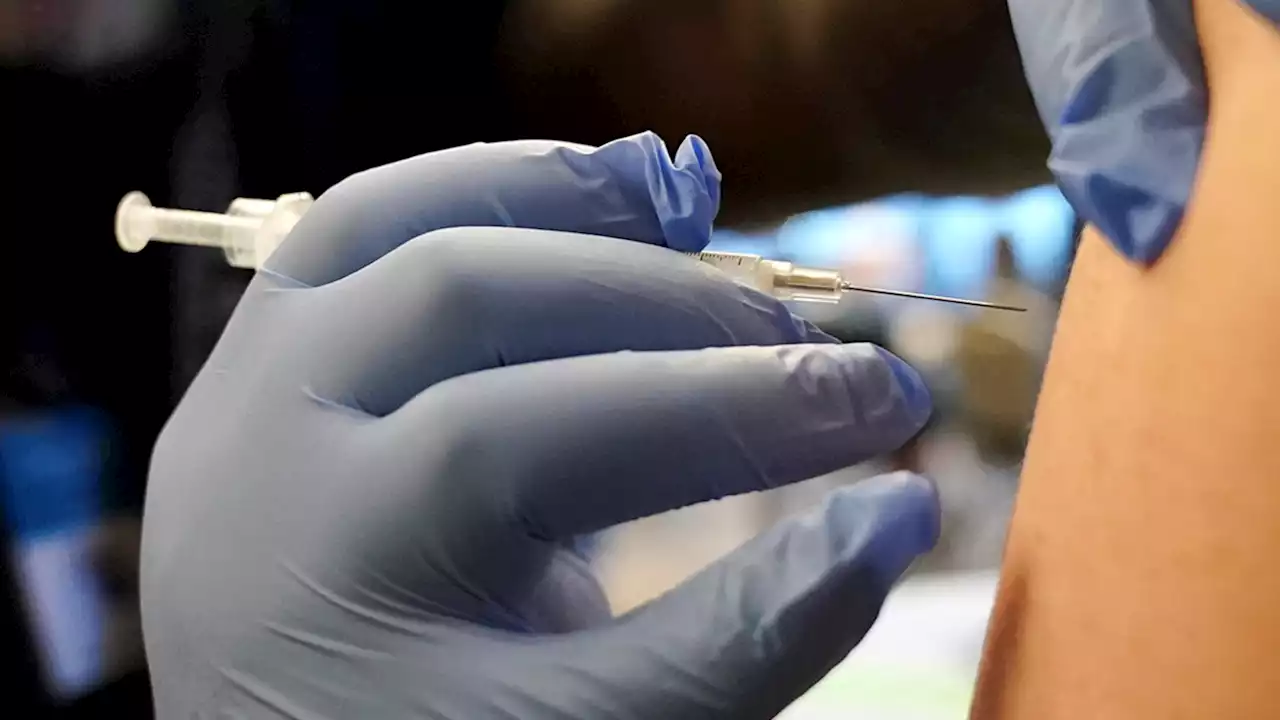 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Leer más »
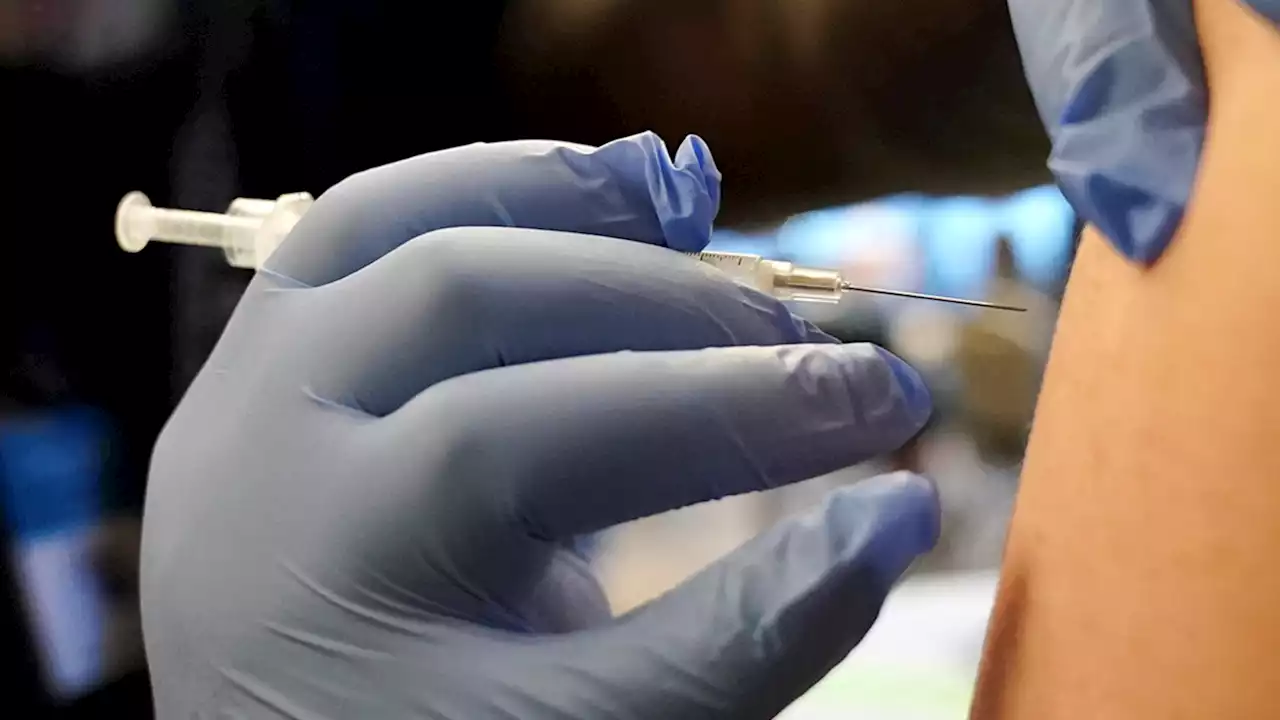 Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccineThe FDA has authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Leer más »
 Children as young as 6 months can now receive an updated Covid-19 vaccine | CNNThe US Food and Drug Administration on Thursday authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Children as young as 6 months can now receive an updated Covid-19 vaccine | CNNThe US Food and Drug Administration on Thursday authorized updated Covid-19 vaccines from Moderna and Pfizer/BioNTech for use in children from ages 6 months through 5 years.
Leer más »
 New Research: Your Choice of COVID Vaccine Can Increase Your Risk of MyocarditisThe study found that Moderna had greater rates of heart inflammation than Pfizer, although the overall risk remained extremely low. In comparison to the Pfizer BioNTech COVID-19 vaccine, the Moderna Spikevax COVID-19 vaccine has a two- to three-fold greater incidence of myocarditis, pericarditis, o
New Research: Your Choice of COVID Vaccine Can Increase Your Risk of MyocarditisThe study found that Moderna had greater rates of heart inflammation than Pfizer, although the overall risk remained extremely low. In comparison to the Pfizer BioNTech COVID-19 vaccine, the Moderna Spikevax COVID-19 vaccine has a two- to three-fold greater incidence of myocarditis, pericarditis, o
Leer más »
