Health experts are supporting the FDA's decision to limit the use of two monoclonal antibody treatments after reports that they were ineffective against Omicron.
Naval Medical Center San Diego administers monoclonal antibody treatment to a COVID-19-positive patient.On Monday evening, the Food and Drug Administration announced that it would limit the use of two monoclonal antibody COVID-19 treatments, made by pharmaceutical companies Regeneron and Eli Lilly. Those treatments had been successful at keeping symptomatic patients out of the hospital in earlier waves,. A third, less common monoclonal treatment, called sotrovimab, can still be used.
For much of early 2021, supplies of the drug were limited, and they were given only to people at high risk of severe COVID. But by this fall, the federal governmenttens to hundred of thousands of doses per week. Some states, including Louisiana and Florida, opened state-run infusion centers where anyone could come in to receive treatment.
, takes at least a few days, but often stretches into a week—by which time, the window for antibody treatment has passed. And most of the sequencing is currently done at a lower standard than the FDA requires for clinical decision-making. PCR tests used by some healthcare institutions could detect one telltale mutation on Omicron, while others, like Houston Methodist Hospital in Texas, rolled out a test that could distinguish between strains as Omicron spread.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
Leer más »
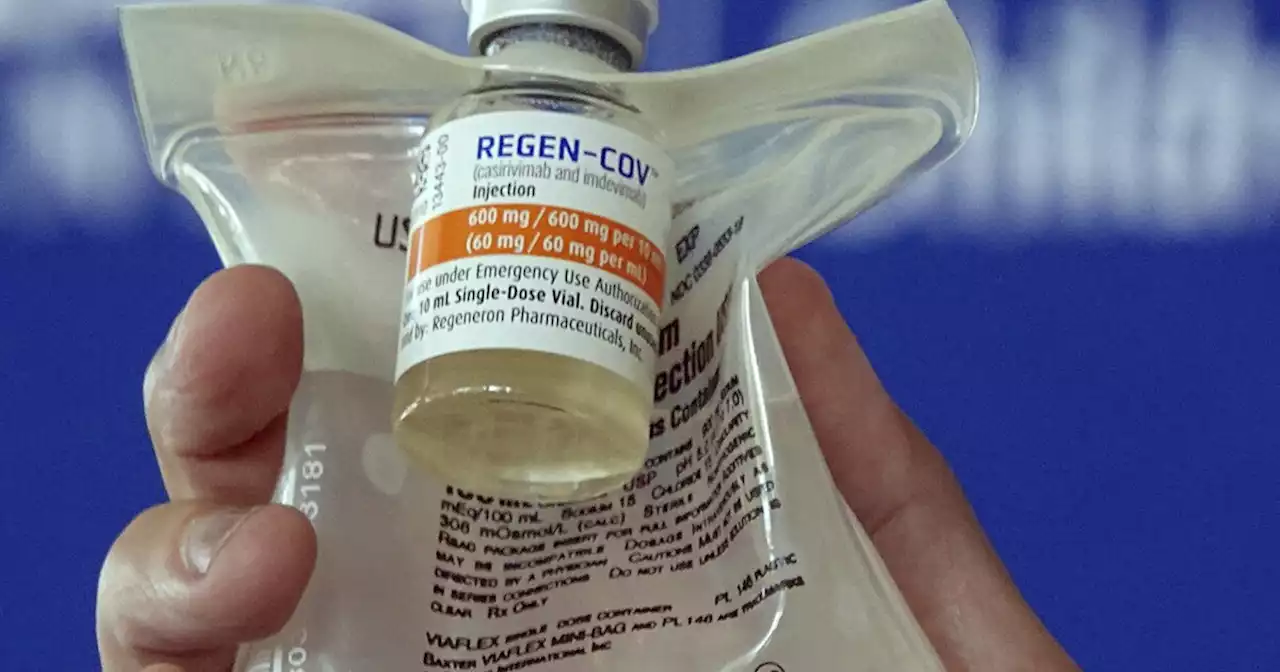 FDA halts use of antibody drugs that don't work against OmicronU.S. officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against Omicron variant.
FDA halts use of antibody drugs that don't work against OmicronU.S. officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against Omicron variant.
Leer más »
 Jen Psaki Calls DeSantis ‘Crazy’ For Pushing To Reverse FDA Halt On Covid Antibody TreatmentsI am a Greece-born reporter covering breaking news. Follow me on Twitter BKimNews or email me at lkim[at]forbes[dot]com
Jen Psaki Calls DeSantis ‘Crazy’ For Pushing To Reverse FDA Halt On Covid Antibody TreatmentsI am a Greece-born reporter covering breaking news. Follow me on Twitter BKimNews or email me at lkim[at]forbes[dot]com
Leer más »
 Rand Paul concocts a new FDA plot to 'punish' conservatives.MaddowBlog: As Sen. Paul sees it, federal officials are deliberately limiting access to Covid treatments as part of a scheme to 'punish' conservatives. That's not at all what's happening.
Rand Paul concocts a new FDA plot to 'punish' conservatives.MaddowBlog: As Sen. Paul sees it, federal officials are deliberately limiting access to Covid treatments as part of a scheme to 'punish' conservatives. That's not at all what's happening.
Leer más »
