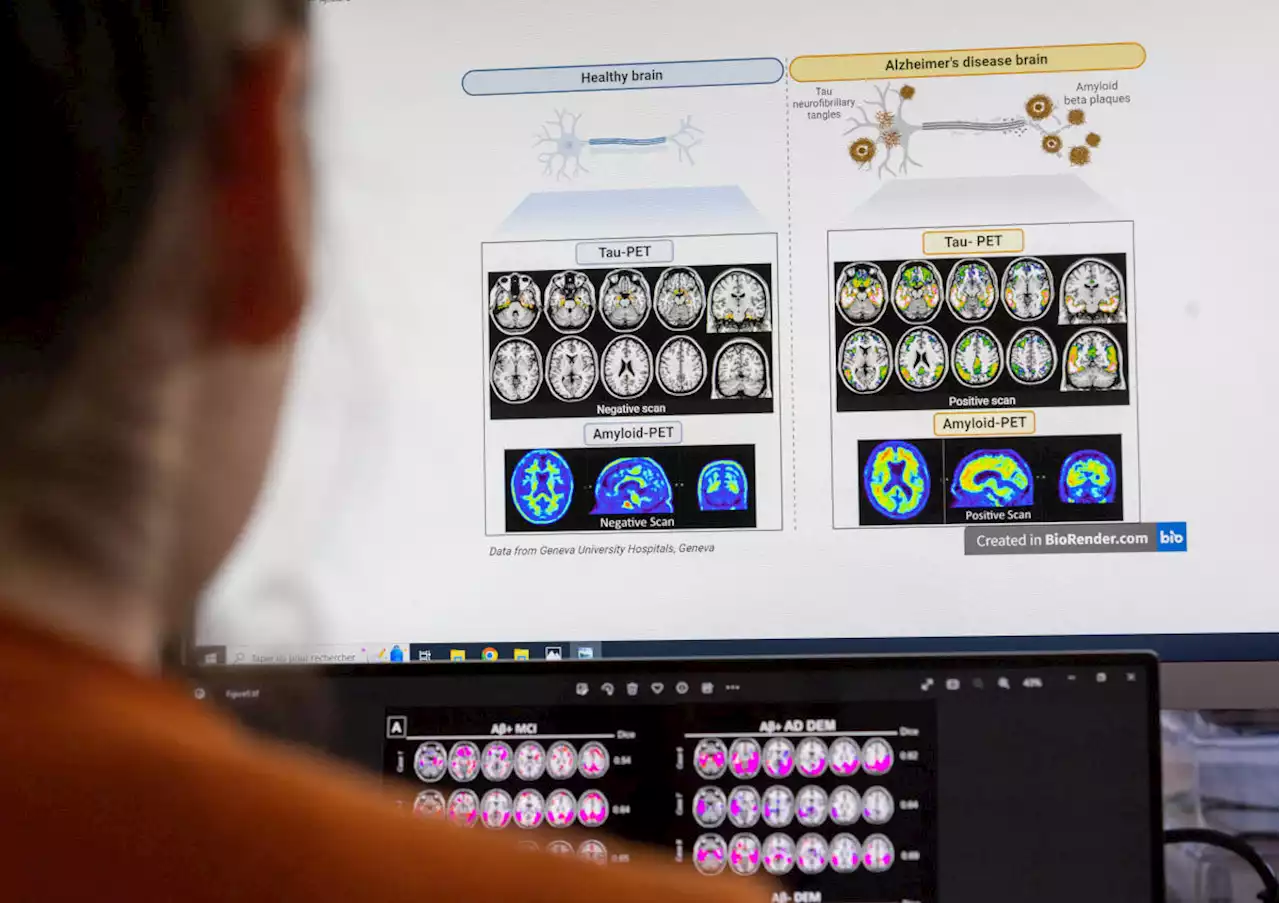
The FDA gave full approval to the drug Leqembi for patients who are in the early stages of Alzheimer’s disease. Here's what you need to know.
On Thursday, the Food and Drug Administration gave full approval to the drug Leqembi for patients who are in the early stages of Alzheimer’s disease, and Medicare said it would cover 80% of the cost of the $26,500-per-year medication. The decisions by the two federal agencies will vastly increase access to the drug but also present a dilemma for patients and their families.
How the drug might affect a patient’s daily life is likely to vary widely. For some people, Leqembi might mean several additional months of being able to follow a recipe, balance a checkbook or accomplish other activities without help. For others, the impact might be much more subtle and barely noticeable.Yes. The drug can cause swelling or bleeding in the brain that is often mild or moderate and resolves on its own but can be serious and in very rare cases can be fatal.
About 17% of the patients receiving Leqembi experienced brain bleeding, compared with 9% of patients receiving the placebo. The most common symptom from brain bleeds was dizziness, the study said.Leqembi — which is administered by intravenous infusions in a doctor’s office or clinic every two weeks — will be available for people diagnosed as having early-stage Alzheimer’s and for those with a pre-Alzheimer’s condition called mild cognitive impairment. About 1.
There are potentially tens of thousands of dollars of additional costs, however — including medical visits for the infusions and regular brain scans. Some Alzheimer’s experts have estimated that the total cost of taking Leqembi could run to about $90,000 a year. With 80% coverage, treatment could potentially leave patients saddled with $18,000 per year in out-of-pocket costs.Talk to your doctor. If your doctor is not well-versed in Alzheimer’s treatments, consider talking with a specialist.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA approves Alzheimer’s drug Leqembi, paving way for broader Medicare coverageLeqembi slowed cognitive decline in a clinical trial, but the treatment is expensive and carries serious risks of brain swelling and bleeding.
FDA approves Alzheimer’s drug Leqembi, paving way for broader Medicare coverageLeqembi slowed cognitive decline in a clinical trial, but the treatment is expensive and carries serious risks of brain swelling and bleeding.
Leer más »
 Alzheimer’s drug Leqembi granted full FDA approvalUPDATE: U.S. officials granted full approval to a closely watched Alzheimer’s drug on Thursday, clearing the way for Medicare and other insurance plans to begin covering the treatment for people with the brain-robbing disease.
Alzheimer’s drug Leqembi granted full FDA approvalUPDATE: U.S. officials granted full approval to a closely watched Alzheimer’s drug on Thursday, clearing the way for Medicare and other insurance plans to begin covering the treatment for people with the brain-robbing disease.
Leer más »
 Alzheimer’s drug Leqembi receives full FDA approvalThe Food and Drug Administration endorsed the IV drug, Leqembi, for patients with mild dementia and other symptoms caused by early Alzheimer’s disease. It’s the first medicine that’s been convincingly shown to modestly slow Alzheimer’s cognitive decline.
Alzheimer’s drug Leqembi receives full FDA approvalThe Food and Drug Administration endorsed the IV drug, Leqembi, for patients with mild dementia and other symptoms caused by early Alzheimer’s disease. It’s the first medicine that’s been convincingly shown to modestly slow Alzheimer’s cognitive decline.
Leer más »
 FDA Approves Promising New Alzheimer’s Drug LeqembiNot only is it the first Alzheimer’s antibody treatment to get full FDA approval, it’s also the first that will be broadly covered by Medicare.
FDA Approves Promising New Alzheimer’s Drug LeqembiNot only is it the first Alzheimer’s antibody treatment to get full FDA approval, it’s also the first that will be broadly covered by Medicare.
Leer más »
 Alzheimer's drug Leqembi wins full FDA approvalThis clears the way for Medicare and other insurance plans to begin covering the treatment.
Alzheimer's drug Leqembi wins full FDA approvalThis clears the way for Medicare and other insurance plans to begin covering the treatment.
Leer más »
 First Alzheimer's drug to slow disease, Leqembi, gets full FDA approvalLeqembi is not a cure, but it is the first drug shown to slow the progression of Alzheimer's disease. It first received an accelerated approval from the FDA earlier this year.
First Alzheimer's drug to slow disease, Leqembi, gets full FDA approvalLeqembi is not a cure, but it is the first drug shown to slow the progression of Alzheimer's disease. It first received an accelerated approval from the FDA earlier this year.
Leer más »