The nation’s 19 million children under 5 are the only group not yet eligible for vaccination against the coronavirus.
The FDA said Tuesday it will convene a panel of independent researchers and physicians in mid-February to help review the Pfizer data. The agency isn’t required to follow the panelists' advice but their input is a key step in publicly vetting vaccine safety and effectiveness.
The Biden administration has been trying to speed the authorization of COVID-19 shots for children, contending vaccinations are critical for opening schools and day care centers and keeping them open, and for freeing up parents from child care duties so they can go back to work. “What we’re seeing right now is still a lot of hospitalizations and unfortunately some deaths in this age group,” said Dr. Sean O’Leary of the University of Colorado, who is on the AAP's infectious disease committee. If the FDA clears vaccinations for these youngsters, “that’s going to be really important because all of those hospitalizations and deaths essentially are preventable.”
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Leer más »
 Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Children under the age of 5 are the only group not yet eligible for vaccination — but that could change soon.
Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Children under the age of 5 are the only group not yet eligible for vaccination — but that could change soon.
Leer más »
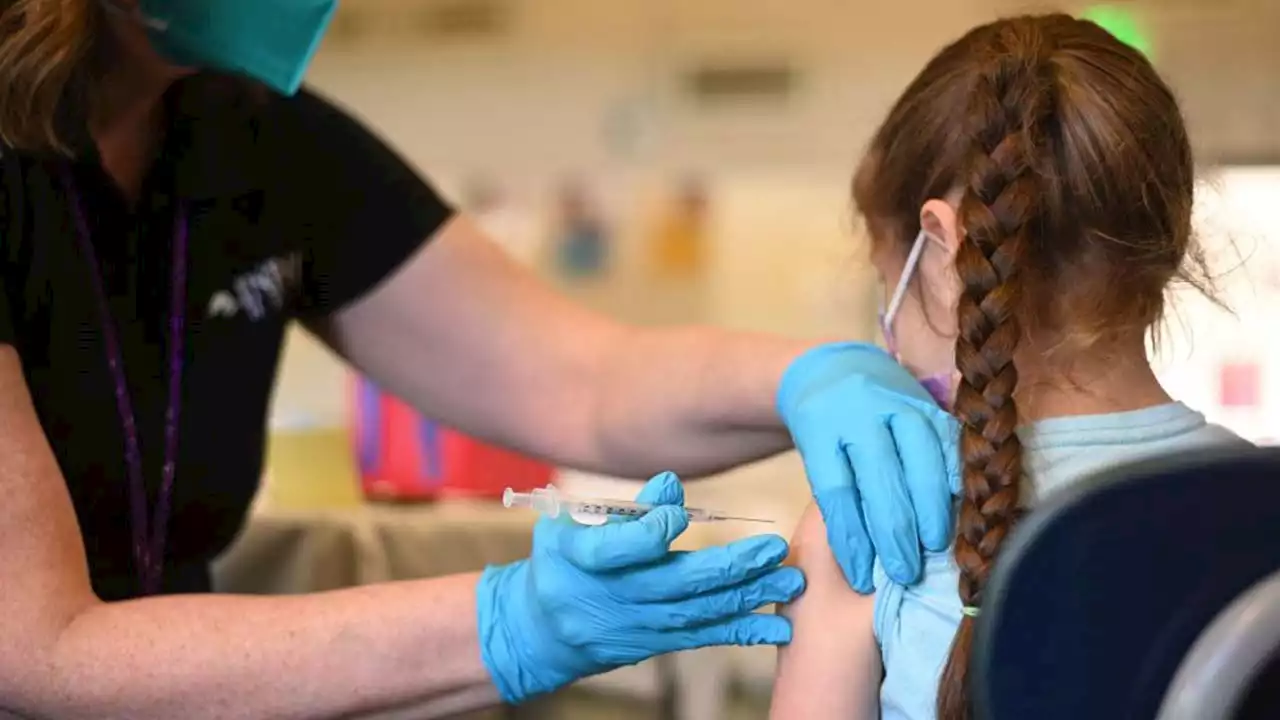 Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Leer más »
 Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Leer más »
 Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Leer más »
