Pfizer Inc and its German partner BioNTech SE said on Monday they have submitted an application to the U.S. Food and Drug Administration (FDA) for emergency use authorization of their Omicron-adapted COVID-19 vaccine booster for children aged 6 months through 4 years.
they have submitted an application to the U.S. Food and Drug Administration for emergency use authorization of their Omicron-adapted COVID-19 vaccine booster for children aged 6 months through 4 years.
If authorized, children would receive the primary series consisting of two doses of the original Pfizer-BioNTech COVID vaccine and one shot of the Omicron-adapted bivalent vaccine, the company said. The bivalent COVID-19 vaccine, which targets the original strain and the BA.4/BA.5 Omicron subvariants, is currently authorized as a booster dose for ages 5 years and older in the United States and the European Union .
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Leer más »
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Leer más »
 Pfizer, BioNTech ask FDA to authorize bivalent COVID vaccine in young childrenPfizer Inc. undefined and BioNTech SE undefined said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as...
Pfizer, BioNTech ask FDA to authorize bivalent COVID vaccine in young childrenPfizer Inc. undefined and BioNTech SE undefined said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as...
Leer más »
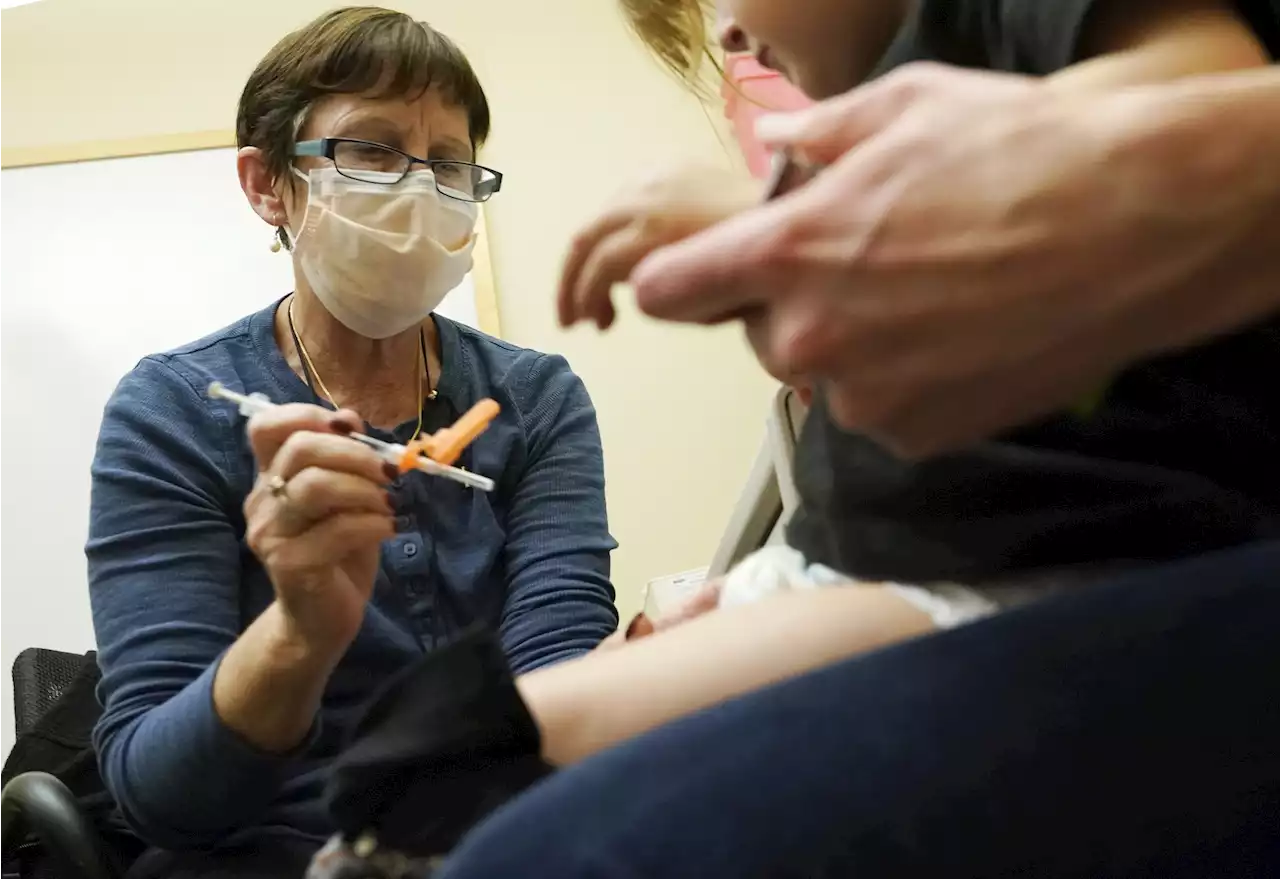 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Leer más »
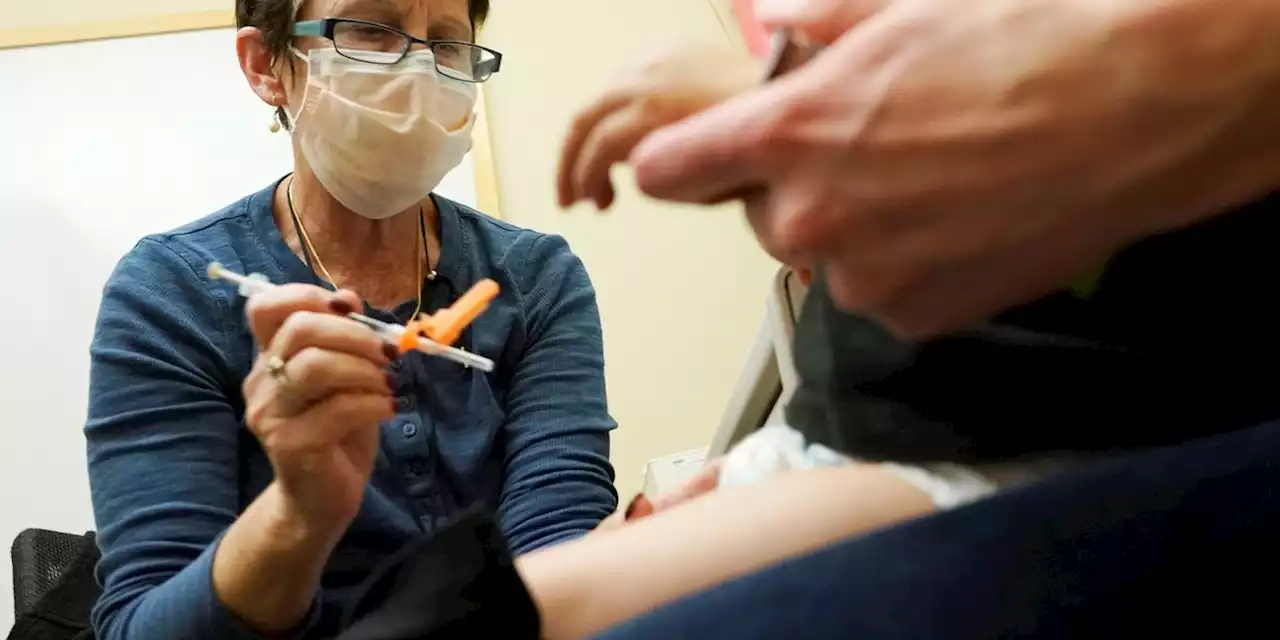 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Leer más »
 Omicron subvariants BQ.1 and BQ.1.1 are now dominant in the U.S. Here's what you need to know.The coronavirus has continued to mutate, and two new Omicron subvariants have now become dominant in the U.S., according to the latest data from the Centers for Disease Control and Prevention. The subvariants, called BQ.1 and BQ.1.1, are descendants of BA.5, the Omicron subvariant that dominated infections in the U.S. since the summer. Together, they now account for more than half of infections nationwide.
Omicron subvariants BQ.1 and BQ.1.1 are now dominant in the U.S. Here's what you need to know.The coronavirus has continued to mutate, and two new Omicron subvariants have now become dominant in the U.S., according to the latest data from the Centers for Disease Control and Prevention. The subvariants, called BQ.1 and BQ.1.1, are descendants of BA.5, the Omicron subvariant that dominated infections in the U.S. since the summer. Together, they now account for more than half of infections nationwide.
Leer más »
