FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
The , companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with– to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said. The inflatable plastic rings are worn around a baby's neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proved.
The floats"have not been evaluated by the FDA and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions," the agency said in the June 28 statement. While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur., the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.FDA:"Do Not Use Baby Neck Floats Due to the Risk of Death or Injury: FDA Safety Communication.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA authorizes pharmacists to prescribe Pfizer's pill to treat COVID-19The Food and Drug Administration announced Wednesday that state-licensed pharmacists can now prescribe Paxlovid.
FDA authorizes pharmacists to prescribe Pfizer's pill to treat COVID-19The Food and Drug Administration announced Wednesday that state-licensed pharmacists can now prescribe Paxlovid.
Leer más »
 Bumble Bee smoked clams contaminated with ‘forever chemicals,’ FDA saysBumble Bee's 3.75-ounce cans of smoke clams have been recalled due to the FDA finding harmful levels of chemicals known as 'forever chemicals.'
Bumble Bee smoked clams contaminated with ‘forever chemicals,’ FDA saysBumble Bee's 3.75-ounce cans of smoke clams have been recalled due to the FDA finding harmful levels of chemicals known as 'forever chemicals.'
Leer más »
 If You Have This Bumble Bee Product in Your Pantry, Don't Eat It, FDA WarnsBumble Bee issued a voluntary recall of specific smoked clams due to potential contamination with harmful chemicals.
If You Have This Bumble Bee Product in Your Pantry, Don't Eat It, FDA WarnsBumble Bee issued a voluntary recall of specific smoked clams due to potential contamination with harmful chemicals.
Leer más »
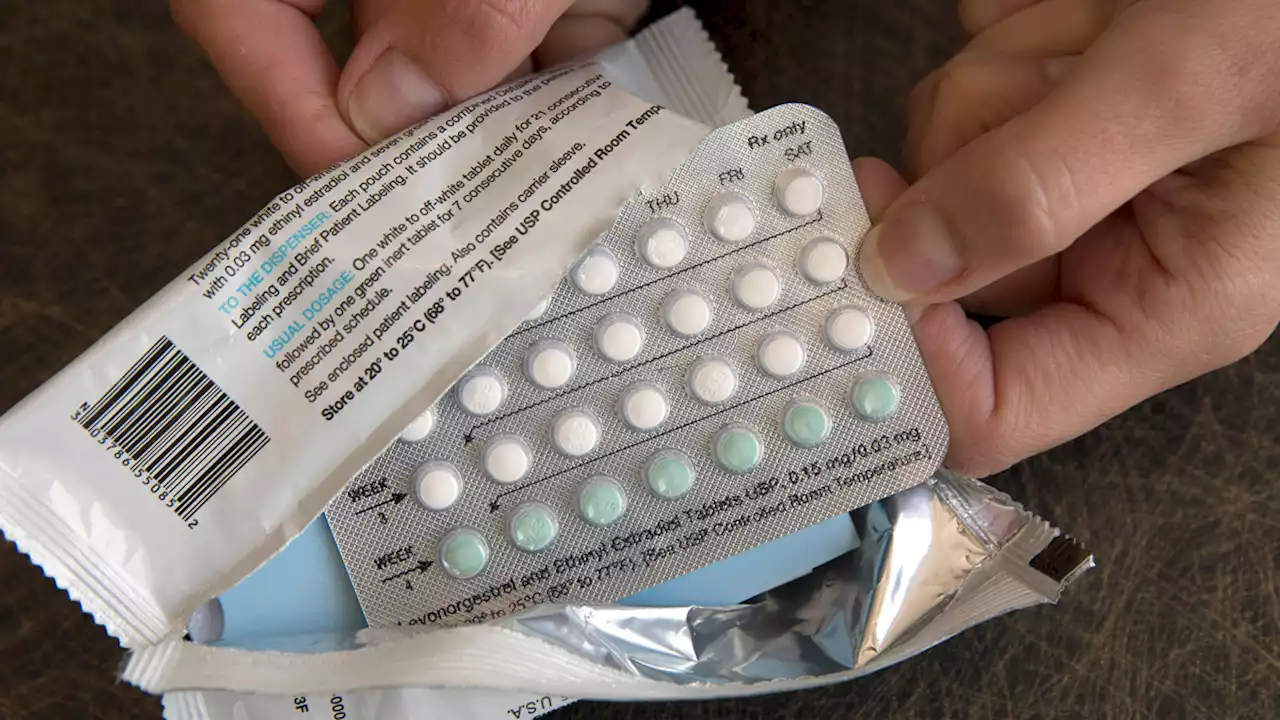 Drugmaker Seeks FDA Approval of First Over-The-Counter Birth Control PillA drug company is seeking U.S. approval for the first-ever birth control pill that women could buy without a prescription
Drugmaker Seeks FDA Approval of First Over-The-Counter Birth Control PillA drug company is seeking U.S. approval for the first-ever birth control pill that women could buy without a prescription
Leer más »
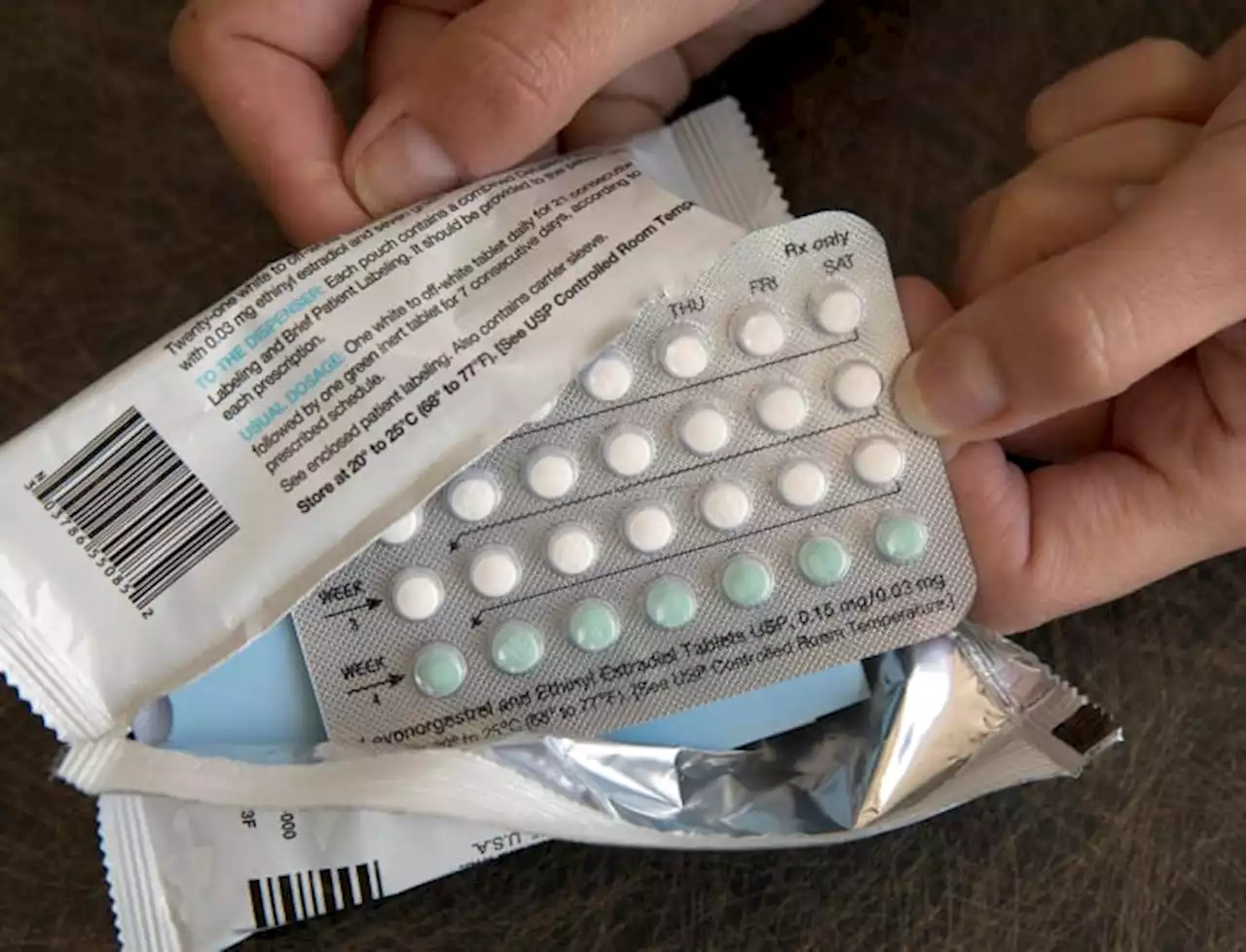 Over-the-counter birth control? Drugmaker seeks FDA approvalA drug company is seeking U.S. approval for the first birth control pill that women could buy without a prescription.
Over-the-counter birth control? Drugmaker seeks FDA approvalA drug company is seeking U.S. approval for the first birth control pill that women could buy without a prescription.
Leer más »
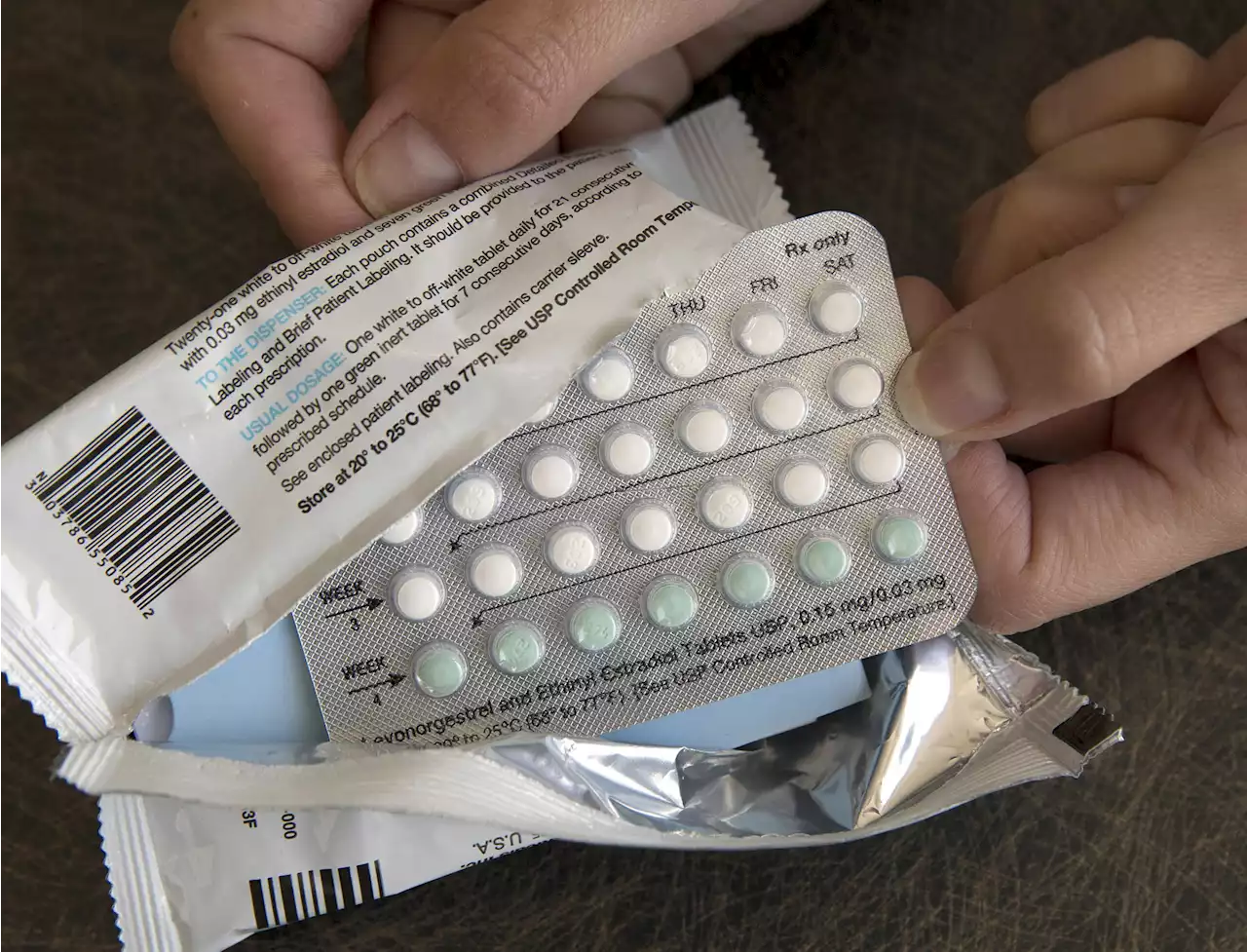 Over-the-counter birth control? Drugmaker seeks FDA approvalWASHINGTON (AP) — For the first time, a pharmaceutical company has asked for permission to sell a birth control pill over the counter in the U.S. HRA Pharma’s application on Monday sets up a high-stakes decision for health regulators amid legal and political battles over women’s reproductive health.
Over-the-counter birth control? Drugmaker seeks FDA approvalWASHINGTON (AP) — For the first time, a pharmaceutical company has asked for permission to sell a birth control pill over the counter in the U.S. HRA Pharma’s application on Monday sets up a high-stakes decision for health regulators amid legal and political battles over women’s reproductive health.
Leer más »
