Moderna is asking U.S. regulators to open its COVID-19 vaccine to the nation's youngest children.
Moderna is asking U.S. regulators to allow for use of its COVID-19 vaccine on the nation's youngest children.
Moderna submitted data to the Food and Drug Administration Thursday for emergency use authorization of its vaccine for children between 6 months and 5 years old. "We believe mRNA-1273 will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19 and will be especially welcomed by parents and caregivers," said Stéphane Bancel, CEO of Moderna.
Kids under 5 are the only group in the U.S. not yet eligible for vaccination and many parents are waiting for a chance to protect them. Pfizer's vaccine is currently available for those 5 and older.It's a complex decision partly because while other countries give Moderna shots to older children, the U.S. so far has restricted them to adults.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Leer más »
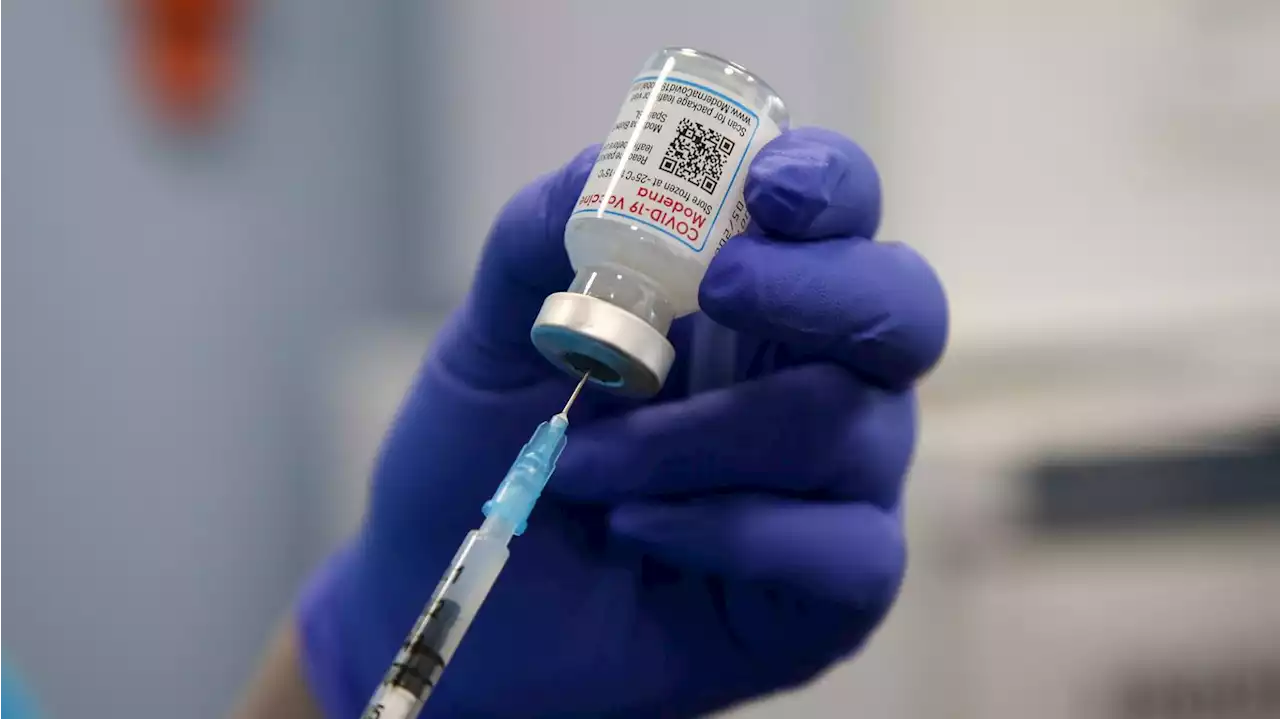 Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Moderna seeks emergency authorization for COVID-19 vaccine in young childrenNEW: Moderna has submitted a request to the FDA for an emergency use authorization for its COVID-19 vaccine in children 6 months to under 6 years of age.
Leer más »
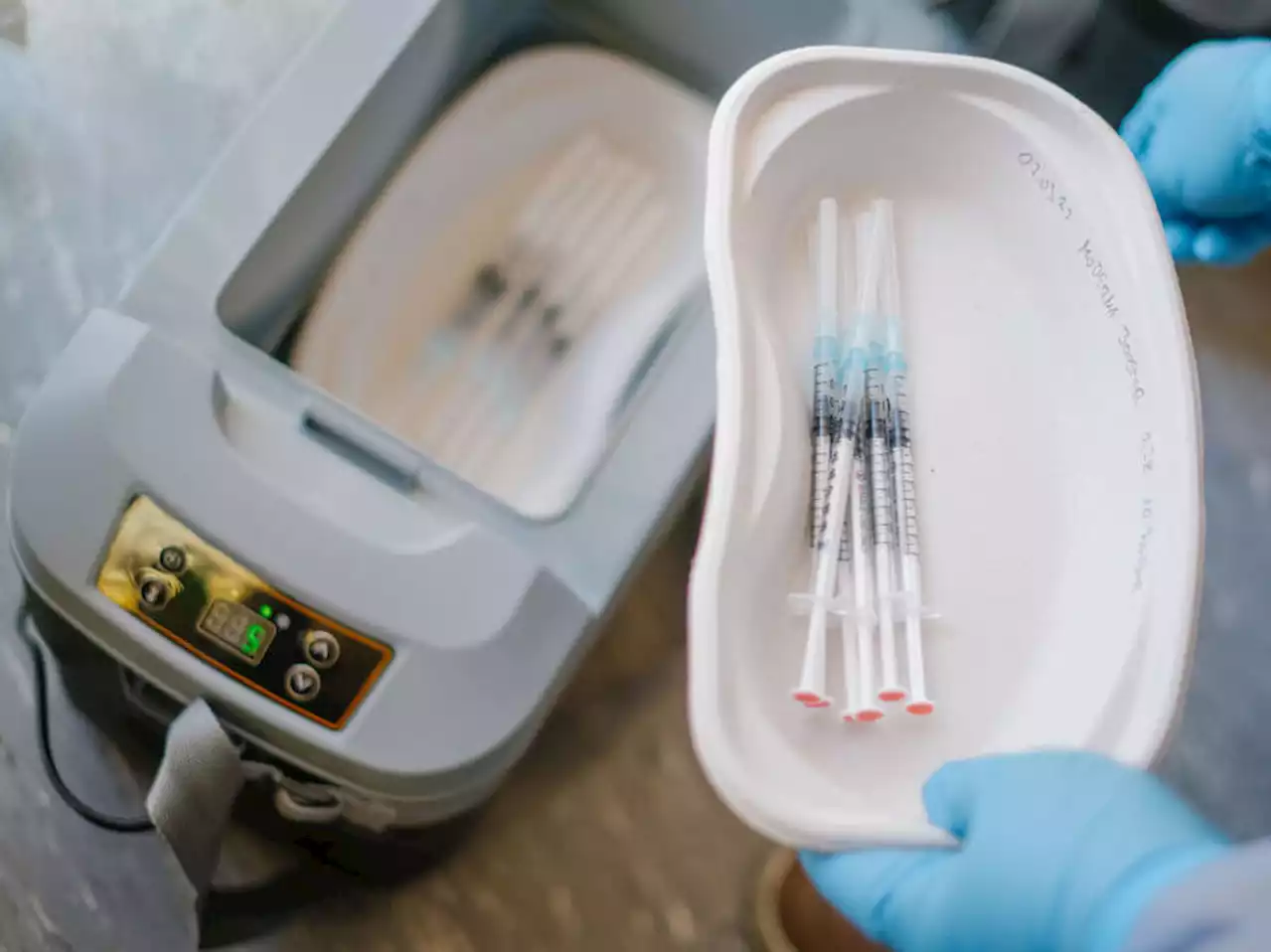 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Leer más »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Leer más »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Leer más »
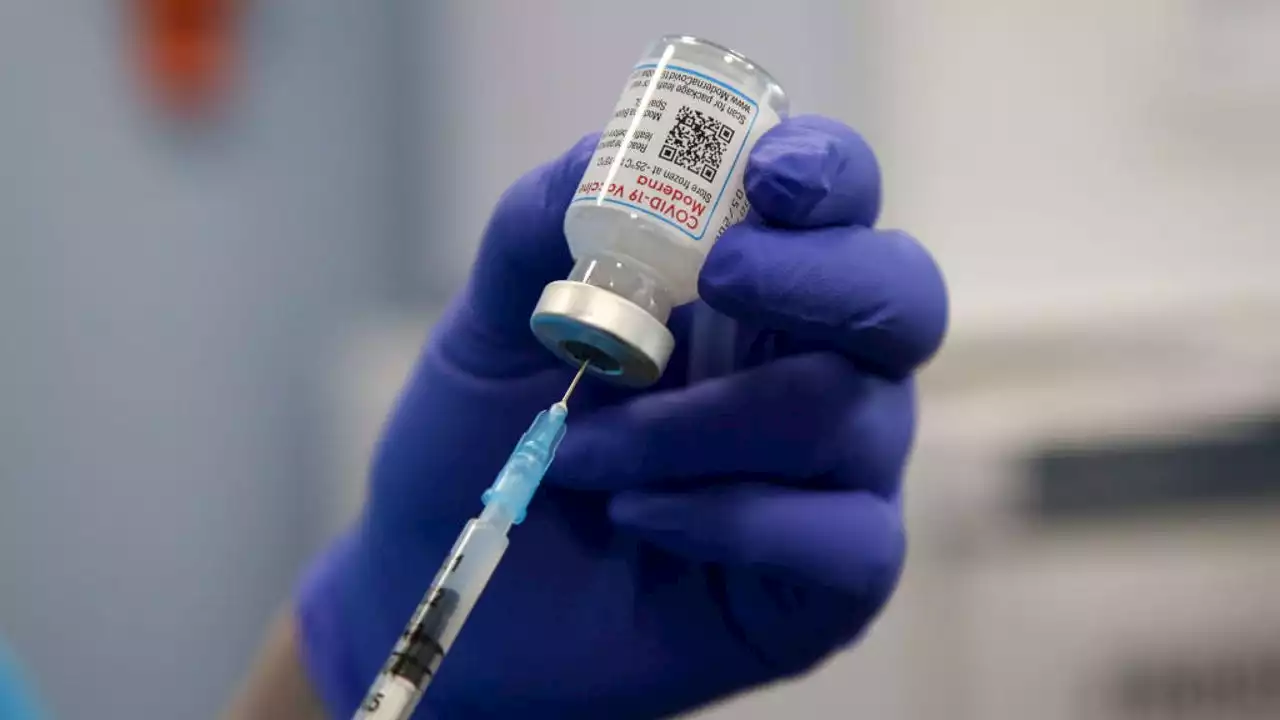 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Leer más »
