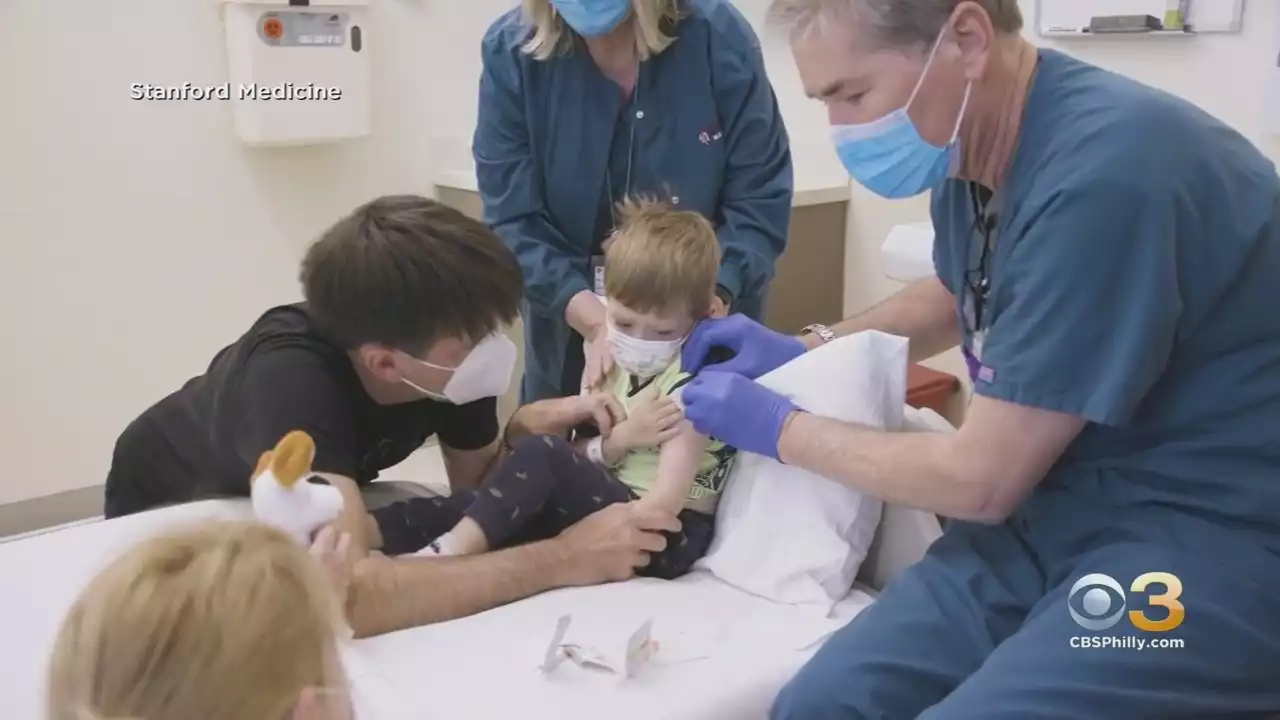
JUST IN: Moderna says its COVID vaccine works for children under 6. It will now seek authorization from the FDA.
“Given the need for a vaccine against COVID-19 in infants and young children we are working with the U.S. FDA and regulators globally to submit these data as soon as possible,” Moderna CEO Stéphane Bancel said. “We believe these latest results … are good news for parents of children under 6 years of age.”The vaccine was not all that effective at preventing Covid-19 infections caused by the Omicron variant, which predominated in the US during the study.
Moderna said it is preparing to evaluate the potential of a booster shot for all children 6 months and older, which would target the original strain of the virus as well as the Omicron variant. The data is based on a group of 6,900 children ages 6 months through 5 years old. The majority of adverse reactions were mild or moderate, and were more frequent after the second shot. Moderna said no deaths and no cases of myocarditis or pericarditis were reported. Myocarditis is inflammation of the heart muscle and pericarditis is inflammation of the heart lining.
Moderna also announced that it has initiated a submission to the FDA for emergency use authorization of the company’s Covid-19 vaccine for children ages 6 through 11 years old. Children that age would get two shots of a larger 50-microgram version of the vaccine. Moderna also said it provided the FDA with additional follow-up data on its vaccine for children ages 12 to 17. Children that age would get two shots of a larger 100-microgram version of the vaccine.
Last month, the FDA postponed a meeting of its vaccine advisers to consider Pfizer/BioNTech’s Covid-19 vaccine for children younger than 5, and requested additional data on third doses. The companies have said they expect that data to be ready by early April.The-CNN-Wire ™ & © 2022 Cable News Network, Inc., a WarnerMedia Company. All rights reserved.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Moderna says its Covid-19 vaccine performs as well in children as it does in adultsModerna announced interim results of its Covid-19 vaccine for children younger than 6 on Wednesday.
Moderna says its Covid-19 vaccine performs as well in children as it does in adultsModerna announced interim results of its Covid-19 vaccine for children younger than 6 on Wednesday.
Leer más »
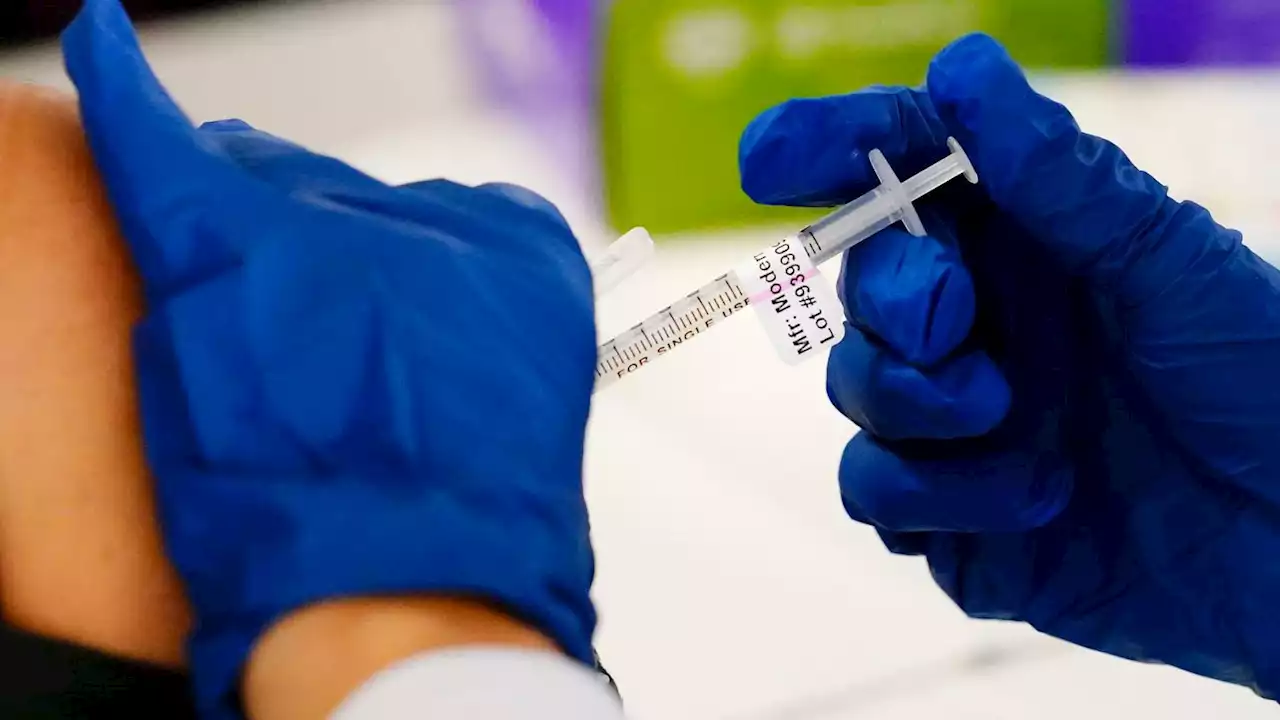 Moderna says low-dose COVID-19 vaccine works in babies, young childrenModerna has announced has a COVID-19 vaccine that works in babies, toddlers and young children.
Moderna says low-dose COVID-19 vaccine works in babies, young childrenModerna has announced has a COVID-19 vaccine that works in babies, toddlers and young children.
Leer más »
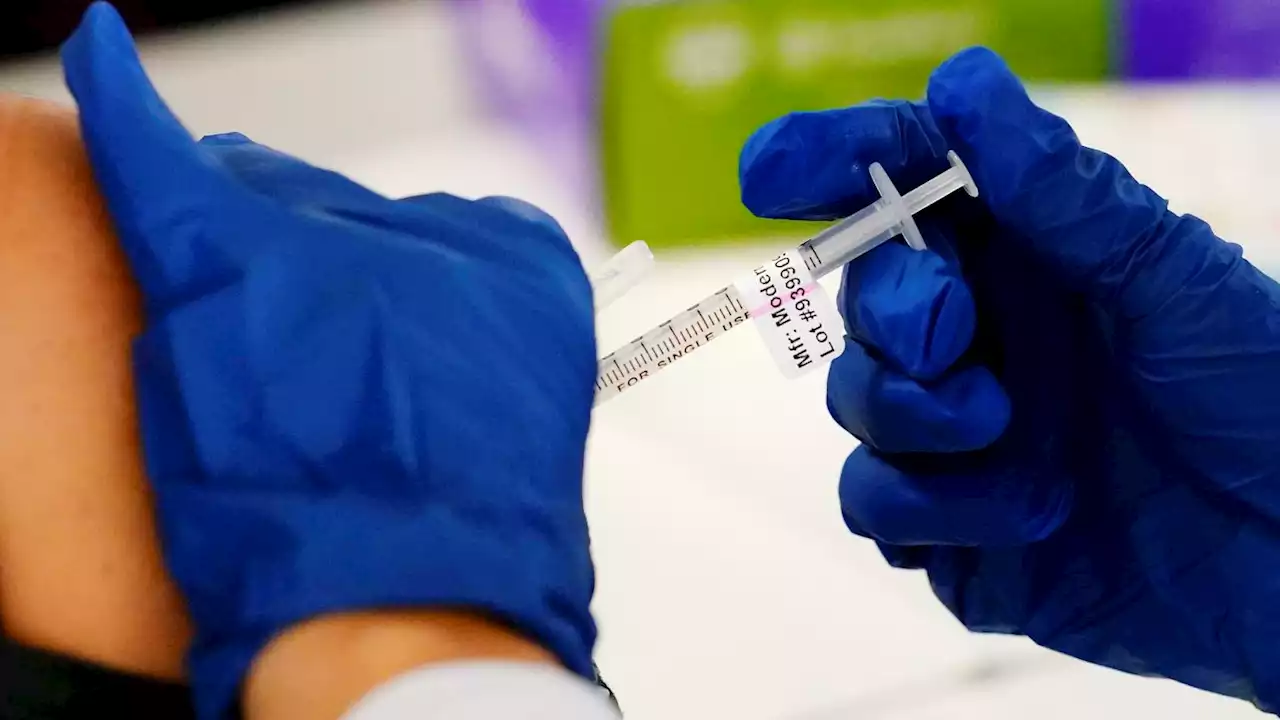 Moderna says its low-dose COVID shots work for kids under 6Moderna says its COVID-19 vaccine works in babies, toddlers and preschoolers
Moderna says its low-dose COVID shots work for kids under 6Moderna says its COVID-19 vaccine works in babies, toddlers and preschoolers
Leer más »
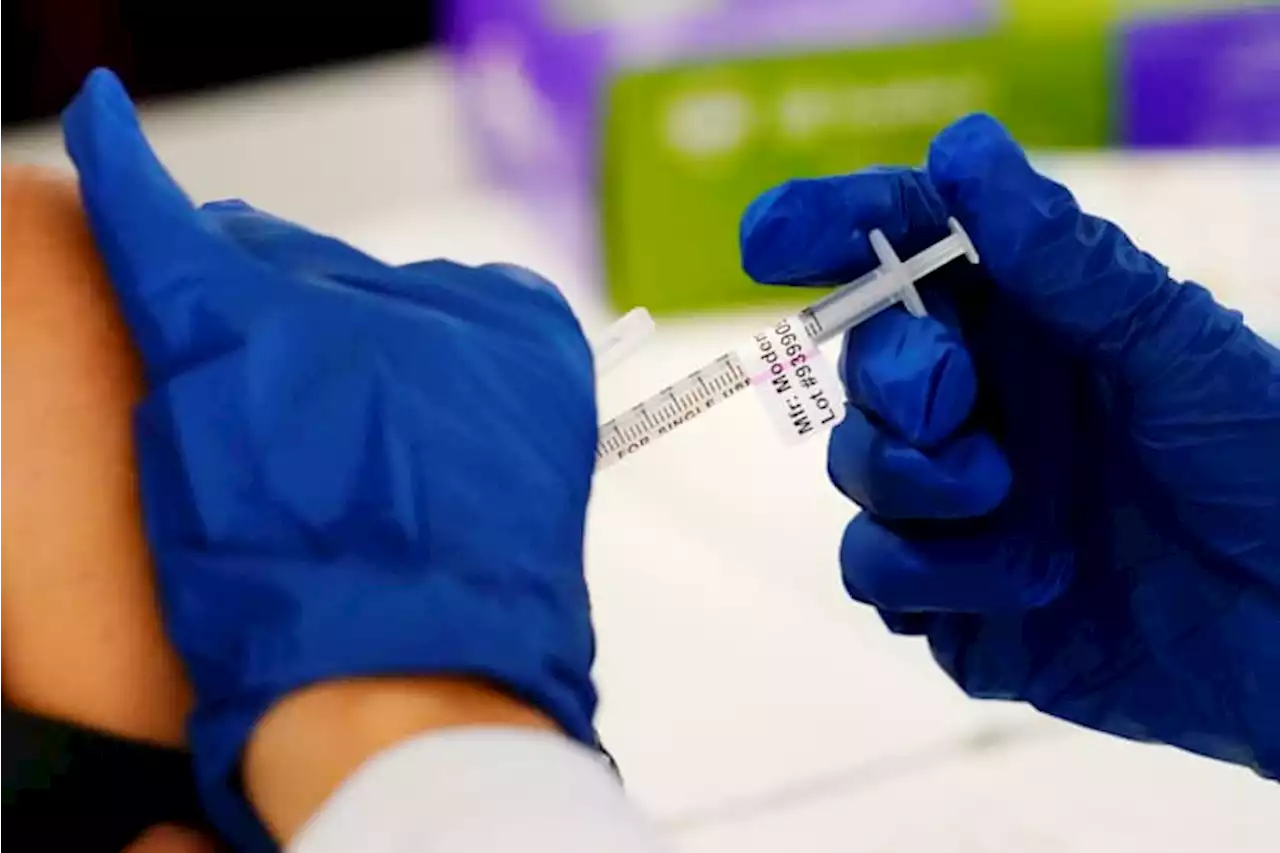 Moderna says its low-dose COVID shots work for kids under 6Moderna said in the coming weeks it would ask regulators in the U.S. and Europe to authorize two small-dose shots for youngsters under 6.
Moderna says its low-dose COVID shots work for kids under 6Moderna said in the coming weeks it would ask regulators in the U.S. and Europe to authorize two small-dose shots for youngsters under 6.
Leer más »
 Moderna expands COVID-19 vaccines to treat related illnessesModerna said Tuesday that it would expand its COVID-19 vaccine to treat related illnesses.
Moderna expands COVID-19 vaccines to treat related illnessesModerna said Tuesday that it would expand its COVID-19 vaccine to treat related illnesses.
Leer más »
NPR Cookie Consent and Choices
Leer más »