BREAKING: Moderna Asks FDA to Authorize COVID Vaccine for Kids Under 6 Years Old
Dr. Peter Marks, who heads the FDA office responsible for vaccines, told the Senate health committee this week that the drug regulator's committee of independent advisors will meet to fully review the data.
"We will proceed with all due speed once we have complete applications," Marks said. He told the committee that the FDA will publish a timeline in the next week for advisory committee meetings on several emergency-use applications. The FDA is in the process of clearing several potential dates for the committee to meet in June, according to a person familiar with matter.
Parents have been waiting for months for a way to protect their children against the virus. During the winter omicron wave, children younger than 5-years-old were hospitalized with Covid at five times the rate of the pandemic's peak, when delta was dominant,About 75% of children under 11 years old had been infected with Covid as of February, according to data released by the CDC this week.
The FDA had originally sought to fast track authorization of Pfizer's Covid vaccine for children under age 5 in February by clearing the first two doses of the three-shot vaccine. However, Pfizer decided to postpone its application and wait for data on the third shot, because the results from the first two doses weren't good enough., said the first two shots only had 30% to 40% efficacy, but he expects the third dose to significantly improve protection.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Leer más »
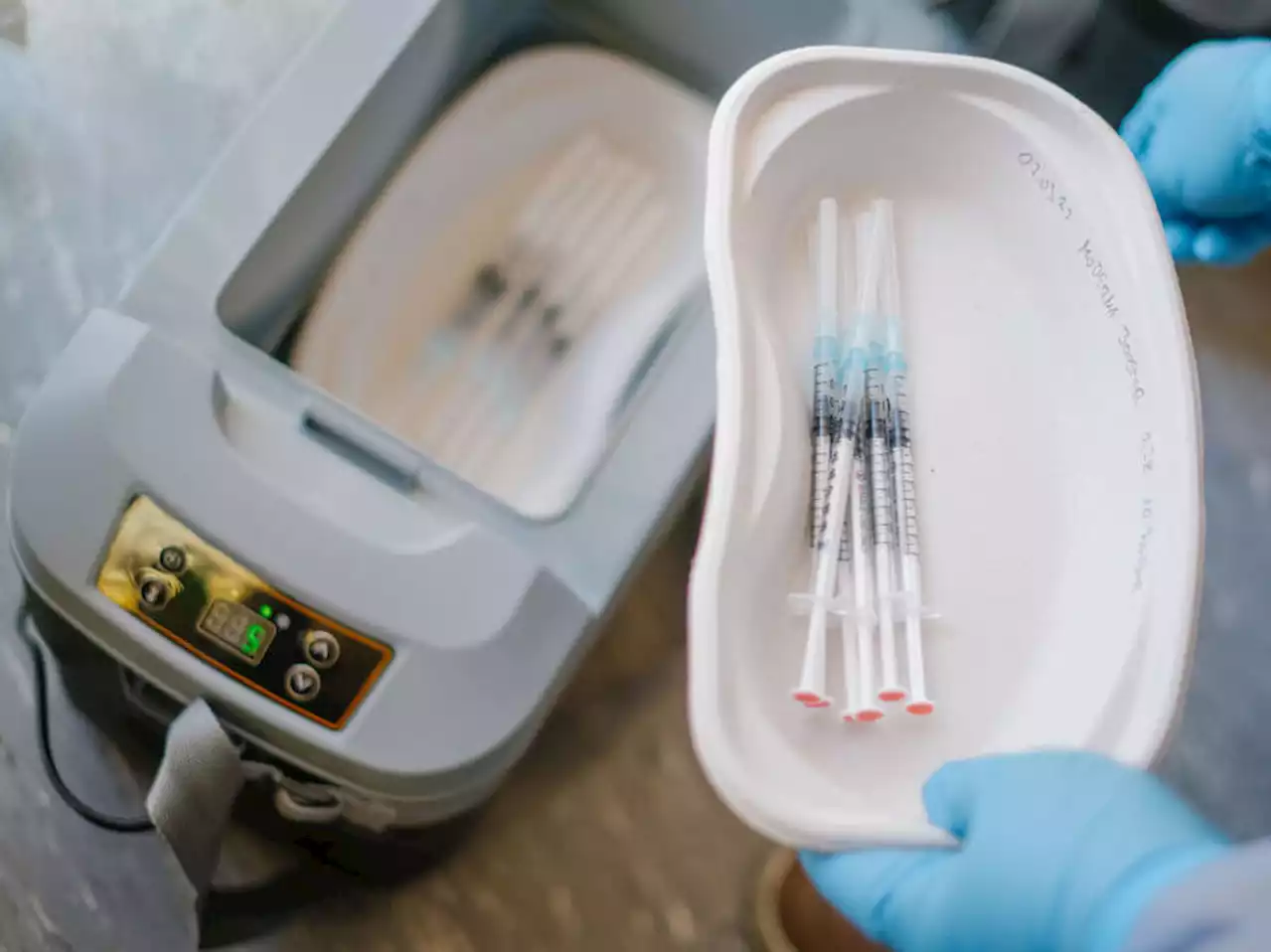 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Leer más »
 Pfizer Asks FDA to Authorize COVID Booster Shots for Ages 5-11Pfizer and BioNTech have applied to the FDA for authorization of their COVID-19 booster shot for children ages 5-11, according to an update from Pfizer.
Pfizer Asks FDA to Authorize COVID Booster Shots for Ages 5-11Pfizer and BioNTech have applied to the FDA for authorization of their COVID-19 booster shot for children ages 5-11, according to an update from Pfizer.
Leer más »
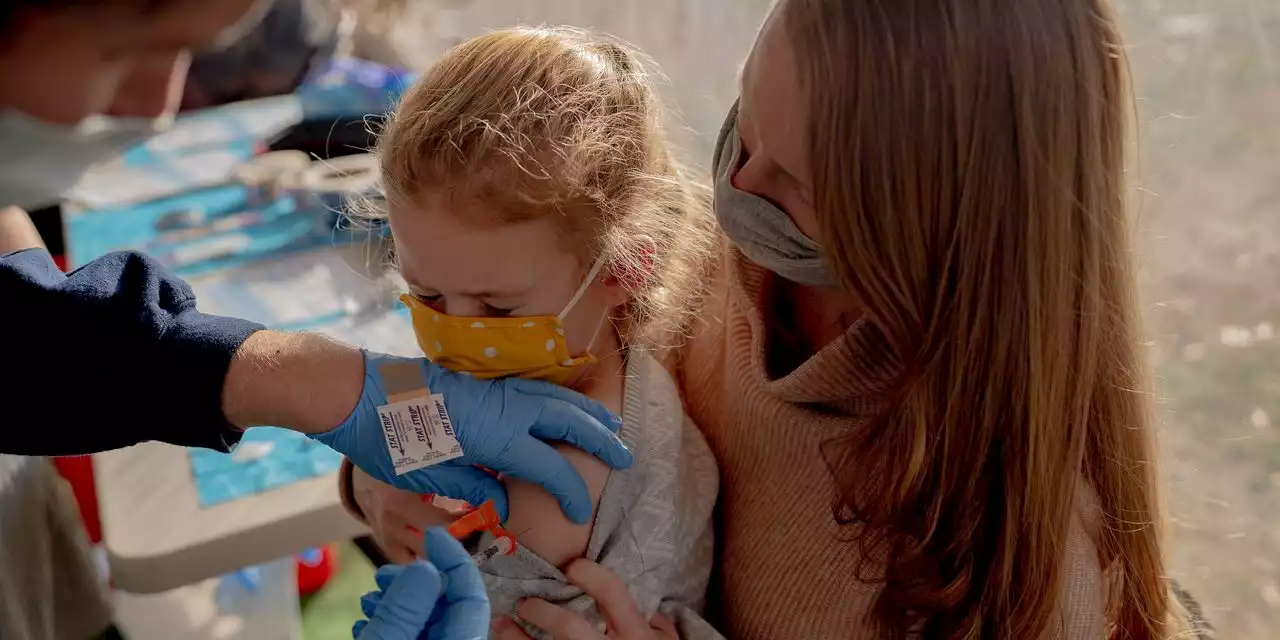 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Leer más »
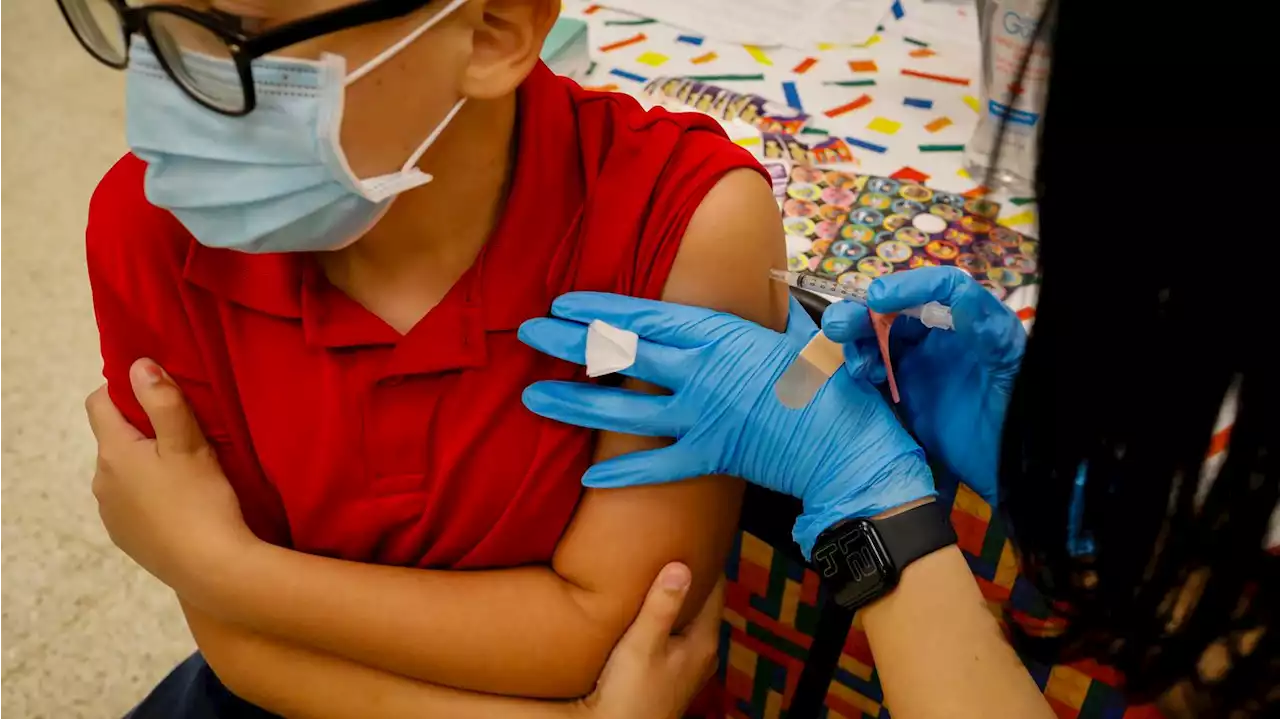 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Leer más »
 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Leer más »
