A global consulting company is under fire for accusations it helped opioid manufacturers make big profits while also consulting for the government agency in charge of regulating those very drugs.
Healey said McKinsey actively worked to advise drug companies to fight off government regulations for addictive prescription drugs.
“The answer to that is quite simply absolutely,” said Healey. “McKinsey was actively coaching Purdue on how to ban together with other opioid companies to fight against those stricter safety requirements.” “It looked at a time period of the work without examining the nature of the work,” said Bob Sternfels, Global Managing Partner for McKinsey & Company. “It implied incorrect conflict standards and it took large speculative leaps to reach unwarranted findings.”
On Tuesday, lawmakers said the FDA announced changes to its contracts with McKinsey as the investigation is underway.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
Leer más »
 Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Leer más »
 FDA Issues Warning About Large Percentage Of False Positives In Prenatal Screening TestsThe FDA emphasized that certain prenatal screening tests only show the risk of certain genetic abnormalities and should not be interpreted as a diagnostic test.
FDA Issues Warning About Large Percentage Of False Positives In Prenatal Screening TestsThe FDA emphasized that certain prenatal screening tests only show the risk of certain genetic abnormalities and should not be interpreted as a diagnostic test.
Leer más »
 FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
Leer más »
 FDA approves first COVID-19 treatment for children under 12 years oldThe FDA gave approval to the use of Veklury (remdesivir) for children 28 days and older who weigh at least 3 kilograms (about 7 pounds).
FDA approves first COVID-19 treatment for children under 12 years oldThe FDA gave approval to the use of Veklury (remdesivir) for children 28 days and older who weigh at least 3 kilograms (about 7 pounds).
Leer más »
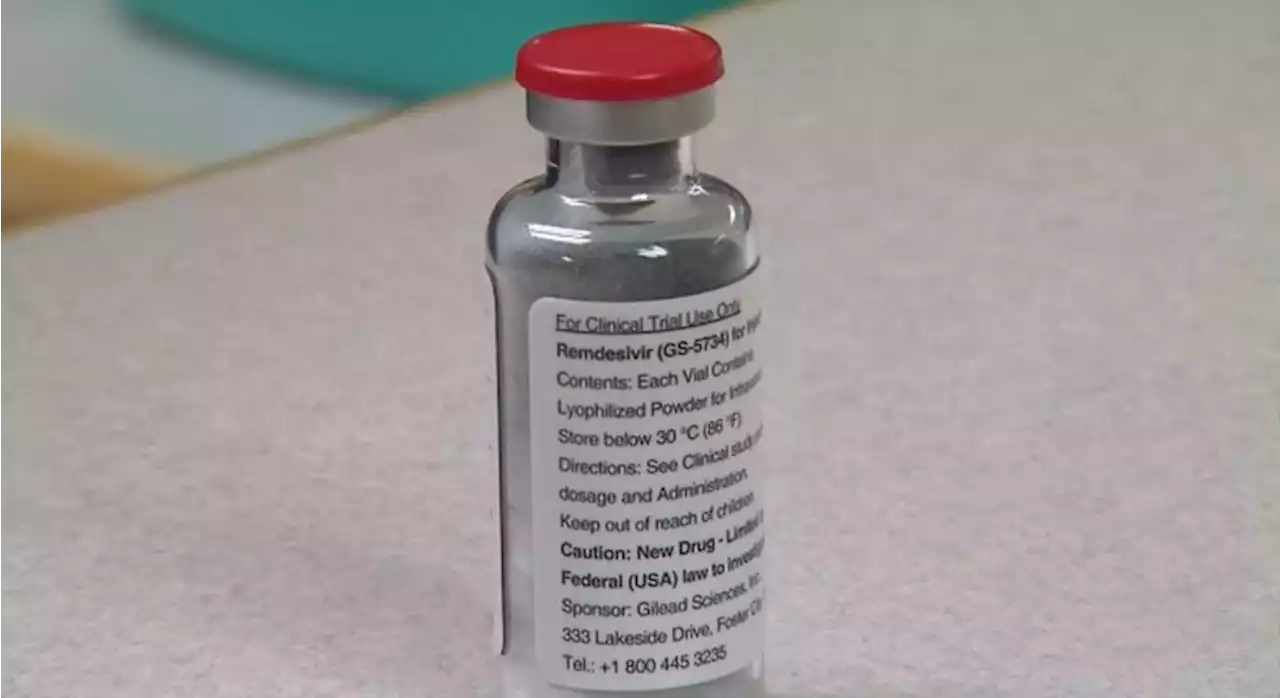 FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
Leer más »