Senate Republicans are demanding answers from the Biden administration about a federal contract signed with KPMG last year to promote monoclonal antibody treatments that they argue did little to forestall shortages of the therapeutics.
The Biden administration entered into a contract worth about $142 million with the accounting firm in March 2021 that was meant to help accelerate access to monoclonal antibody treatments for COVID-19 patients. But several Republicans on the Senate Health Committee, including Tommy Tuberville , argue that the pricey deal did nothing to increase the supplies of the treatments, particularly during a national shortage last year.
The Department of Health and Human Services told the Washington Examiner that the contract was canceled in January 2021 due to the changing uptake and landscape of available treatments in the United States, which now include Pfizer’s antiviral Paxlovid and Merck’s molnupiravir. KPMG spokesman Russ Grote told the Washington Examiner that “KPMG is proud of our work to support all levels of government, health systems, provider networks, and more to provide greater access to life-saving treatments for underserved and disadvantaged communities during the pandemic.”
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Opinion | Biden Broke His Promise to Support Generic Covid-19 Vaccines for the World'Because the United States wields so much power at the WTO, many public health advocates assumed that if the Biden administration wanted to get a waiver passed, it could.'
Opinion | Biden Broke His Promise to Support Generic Covid-19 Vaccines for the World'Because the United States wields so much power at the WTO, many public health advocates assumed that if the Biden administration wanted to get a waiver passed, it could.'
Leer más »
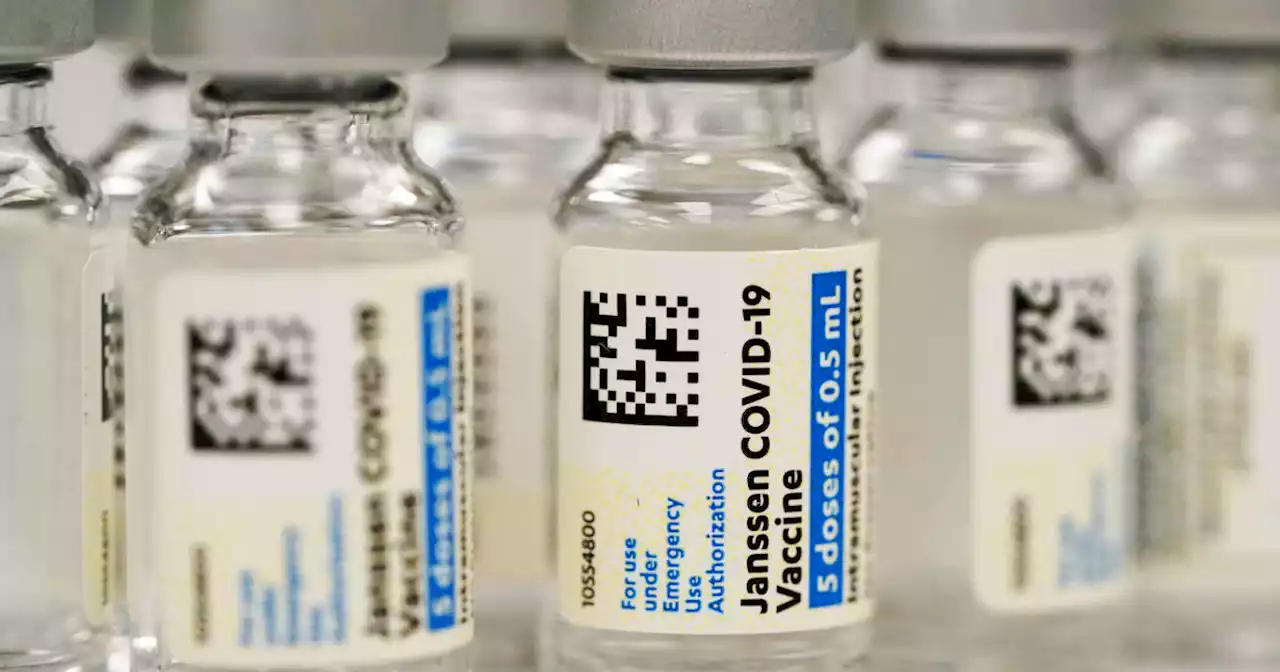 FDA announces limits on who can receive Johnson & Johnson COVID-19 vaccineThe reason is a rare, but serious risk of blood clots.
FDA announces limits on who can receive Johnson & Johnson COVID-19 vaccineThe reason is a rare, but serious risk of blood clots.
Leer más »
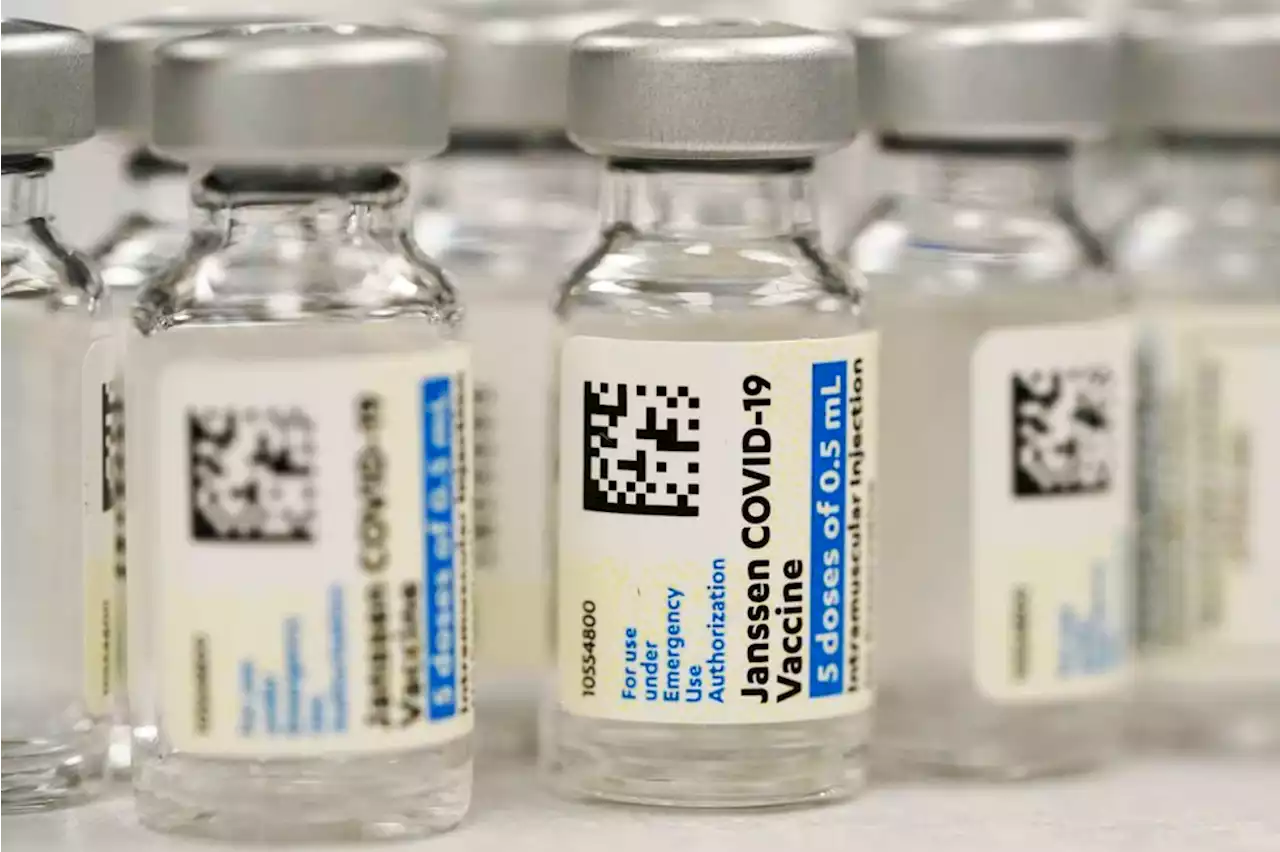 FDA restricts J&J’s COVID-19 vaccine due to blood clot riskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
FDA restricts J&J’s COVID-19 vaccine due to blood clot riskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots.
Leer más »
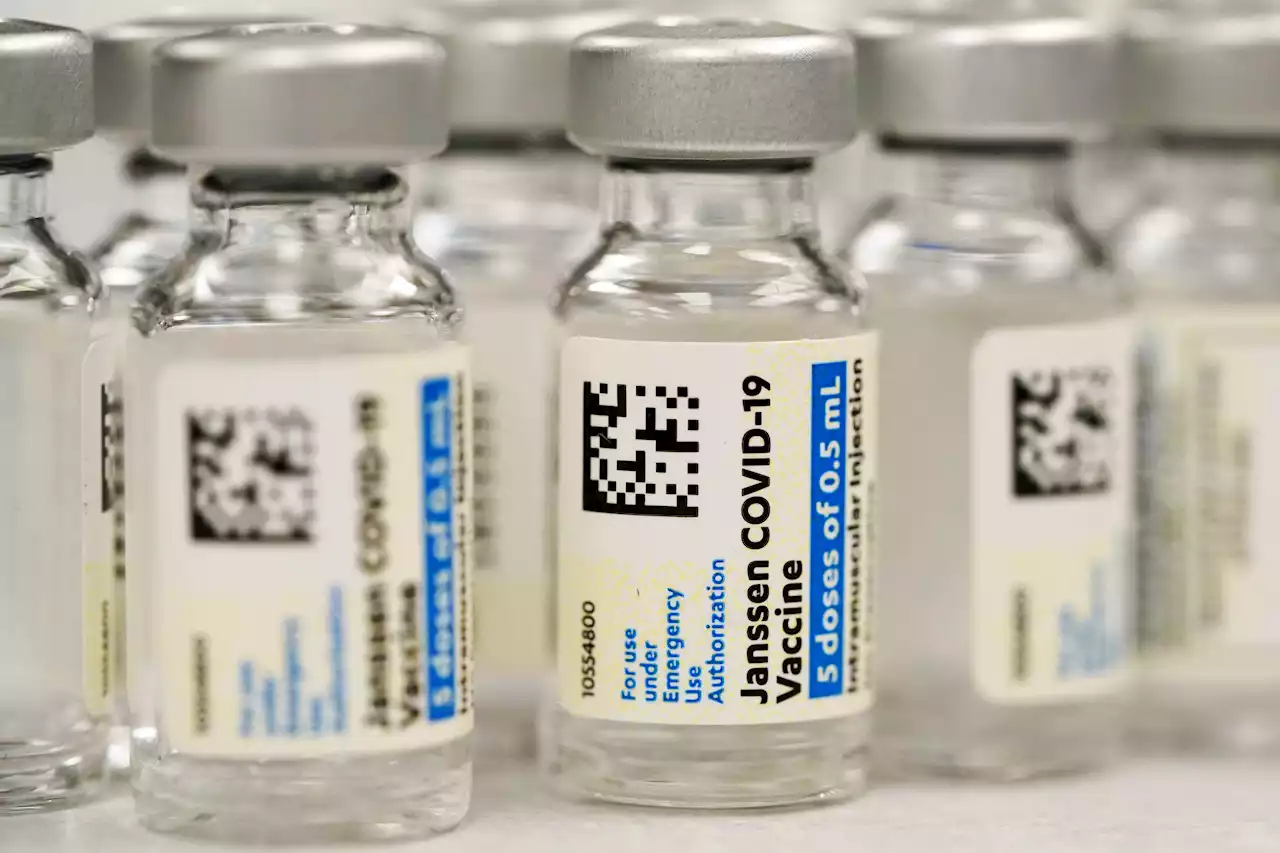 FDA Restricts J&J's COVID-19 Vaccine Due to Blood Clot RiskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots. The Food and Drug Administration said Thursday the shot should only be given to adults who cannot receive a different vaccine or specifically request J&J’s vaccine. The decision is the latest restriction to hit the company’s vaccine, which has long been overshadowed in the U.S. by the more effective shots from Pfizer and Moderna. In December, the Centers for Disease Control and Prevention recommended using the Moderna and Pfizer shots over J&J’s because of its safety issues.
FDA Restricts J&J's COVID-19 Vaccine Due to Blood Clot RiskU.S. regulators are strictly limiting who can receive Johnson & Johnson’s COVID-19 vaccine due to a rare but serious risk of blood clots. The Food and Drug Administration said Thursday the shot should only be given to adults who cannot receive a different vaccine or specifically request J&J’s vaccine. The decision is the latest restriction to hit the company’s vaccine, which has long been overshadowed in the U.S. by the more effective shots from Pfizer and Moderna. In December, the Centers for Disease Control and Prevention recommended using the Moderna and Pfizer shots over J&J’s because of its safety issues.
Leer más »
 Nearly 1 million COVID-19 deaths: A look at the US numbersDoug Lambrecht was among the first of the nearly 1 million Americans to die from COVID-19. His demographic profile — an older white male with chronic health problems — mirrors the faces of many who would be lost over the next two years.
Nearly 1 million COVID-19 deaths: A look at the US numbersDoug Lambrecht was among the first of the nearly 1 million Americans to die from COVID-19. His demographic profile — an older white male with chronic health problems — mirrors the faces of many who would be lost over the next two years.
Leer más »
 Nearly 1 million COVID-19 deaths: A look at the US numbersThe count of U.S. deaths from COVID-19 is nearing 1 million, and there's a wealth of data making clear which groups have been hit the hardest
Nearly 1 million COVID-19 deaths: A look at the US numbersThe count of U.S. deaths from COVID-19 is nearing 1 million, and there's a wealth of data making clear which groups have been hit the hardest
Leer más »
