COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
WASHINGTON —
Omicron’s resistance to the two leading monoclonal antibody medicines has upended the treatment playbook for COVID-19 in recent weeks. The FDA noted in its decision that omicron accounts for more than 99% of U.S. infections, making it “highly unlikely” the antibodies would help people now seeking treatment. The agency said restricting their use would also eliminate unnecessary drug side effects, including allergic reactions.
The drugs are not a substitute for vaccination and are generally reserved for people who are the most vulnerable, including seniors, transplant recipients and those with conditions like heart disease and diabetes. The move comes days after regulators broadened the use of remdesivir — the first drug approved for COVID-19 — to treat more patients.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
Leer más »
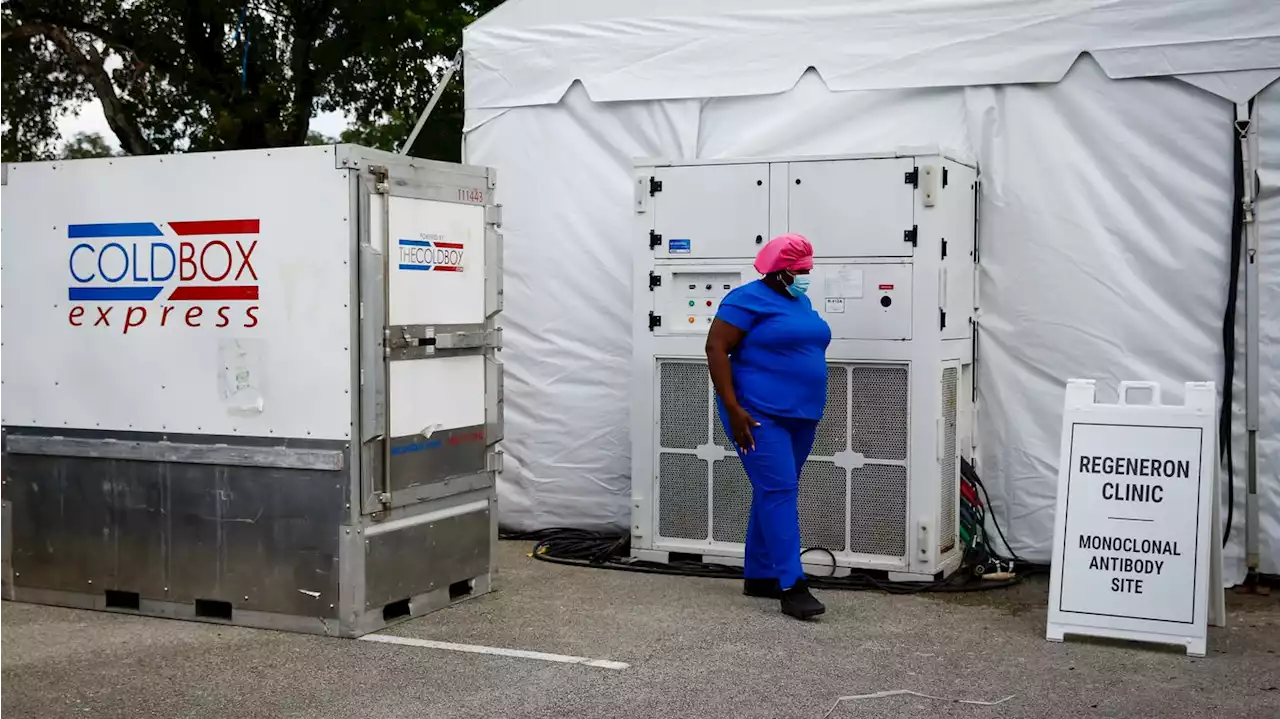 FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
Leer más »
 FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
Leer más »
 FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
Leer más »
 Biden Administration Limits Use of Regeneron and Lilly Covid-19 Antibody DrugsThe FDA revised the authorizations for the therapies, which don’t work against the Omicron variant, to make sure doctors only use them on non-Omicron patients, and the Health and Human Services Department stopped shipments.
Biden Administration Limits Use of Regeneron and Lilly Covid-19 Antibody DrugsThe FDA revised the authorizations for the therapies, which don’t work against the Omicron variant, to make sure doctors only use them on non-Omicron patients, and the Health and Human Services Department stopped shipments.
Leer más »
 Stealth Omicron COVID variant BA.2 that may spread faster found in at least 40 countriesEarly data suggests that the BA.2 Omicron sub-variant, nicknamed 'stealth Omicron,' may have an increased growth rate compared to the variant's dominant form.
Stealth Omicron COVID variant BA.2 that may spread faster found in at least 40 countriesEarly data suggests that the BA.2 Omicron sub-variant, nicknamed 'stealth Omicron,' may have an increased growth rate compared to the variant's dominant form.
Leer más »
