The youngest Americans will have access to variant-targeting boosters already available to older children and adults.
The Food and Drug Administration, in a statement, said children 6 months through 5 years old would be eligible for the booster — known as a “bivalent” shot targeting omicron subvariants BA.4 and BA.5 and the original version of the virus — two months after they have completed Moderna’s two-dose primary series., for children 6 months through 4 years old.
professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine. “It’s like arguing over how many angels can dance on the head of a pin.”But federal health officials, eyeing a rise in covid-19 cases and pediatric hospitalizations because of an array of respiratory germs, believed it was important to make updated shots available to the youngest age groups.
The BA.4 and BA.5 subvariants, which were circulating widely this summer, have faded, and are responsible for less than 15 percent of cases, according to the CDC. They have been replaced byOfficials say the bivalent formulation will offer improved protection against the latest crop of omicron subvariants.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
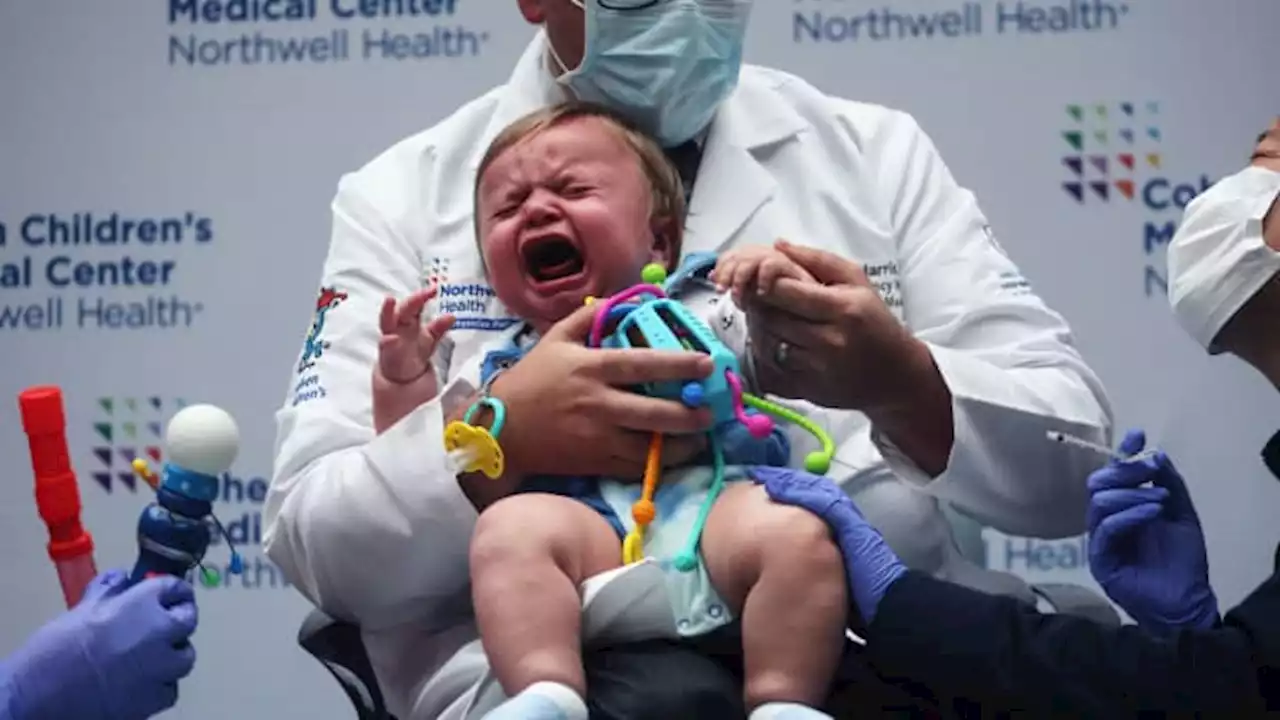 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Leer más »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Leer más »
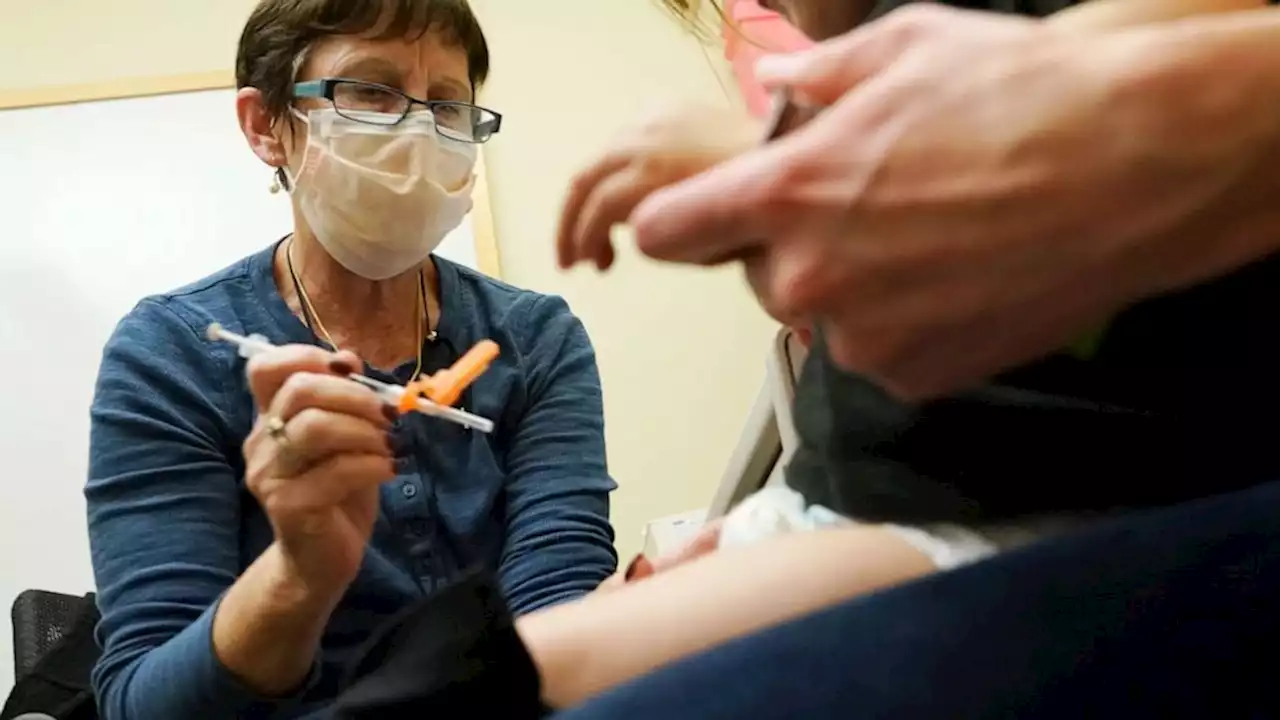 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
Leer más »
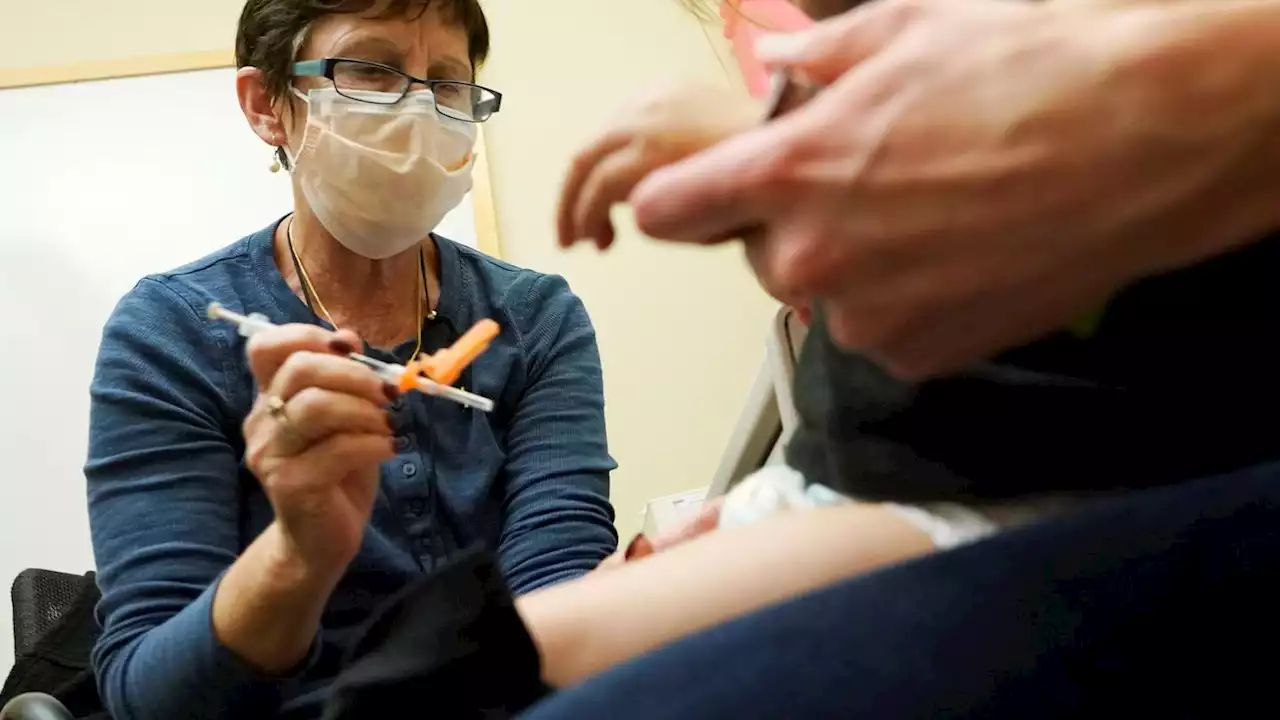 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Leer más »
 Panel calls for stronger leadership of FDA foods programA panel on Tuesday called for changes at the federal agency that oversees most of the nation’s food supply, saying revamped leadership, a clear mission and more urgency are needed to prevent illness outbreaks and to promote good health.
Panel calls for stronger leadership of FDA foods programA panel on Tuesday called for changes at the federal agency that oversees most of the nation’s food supply, saying revamped leadership, a clear mission and more urgency are needed to prevent illness outbreaks and to promote good health.
Leer más »
