U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5. The Food and Drug Administration's decision aims to better protect the littlest kids amid an uptick in COVID-19 cases around the country — at a time when children’s hospitals already are packed with tots suffering from other respiratory illnesses including the flu.
Deborah Sampson, left, a nurse at a University of Washington Medical Center clinic in Seattle, gives a Pfizer COVID-19 vaccine shot to a 20-month-old child, June 21, 2022, in Seattle. The U.S. on Thursday, Dec. 8, 2022 open doses of the updated COVID-19 vaccines for most children younger than age 5.
U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5. “Vaccination is the best way we know to help prevent the serious outcomes of COVID-19, such as hospitalization and death,” Dr. Peter Marks, FDA’s vaccine chief, told The Associated Press.everyone 5 and older.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
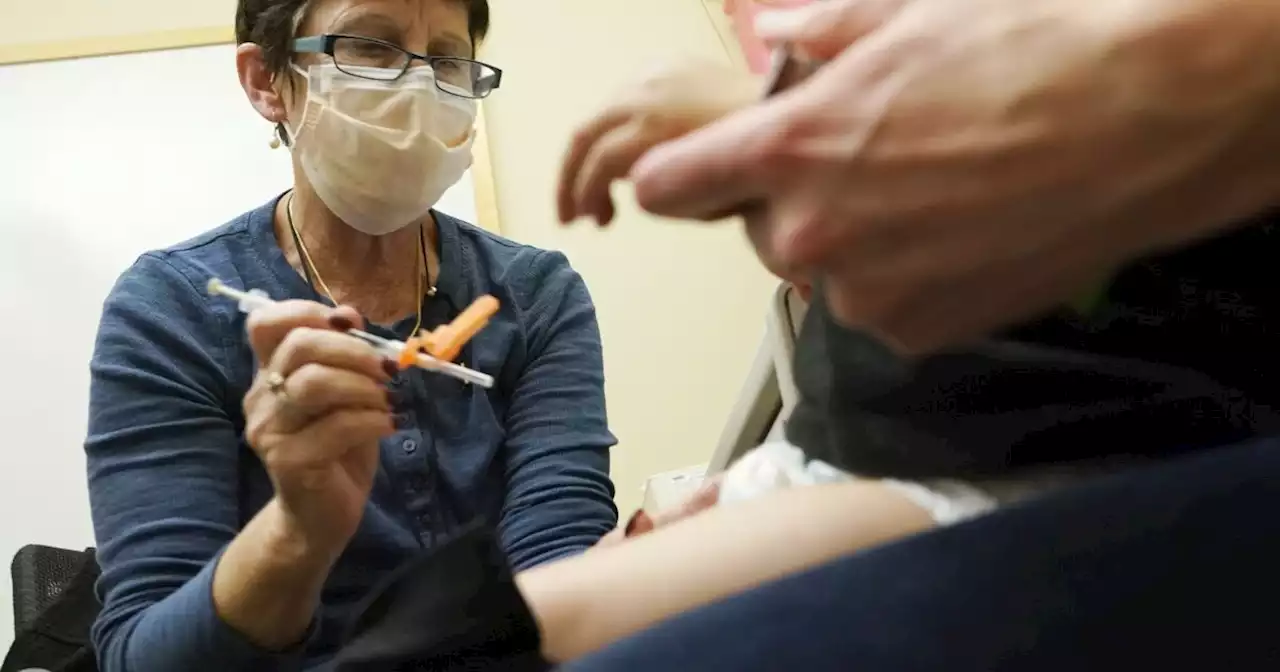 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Leer más »
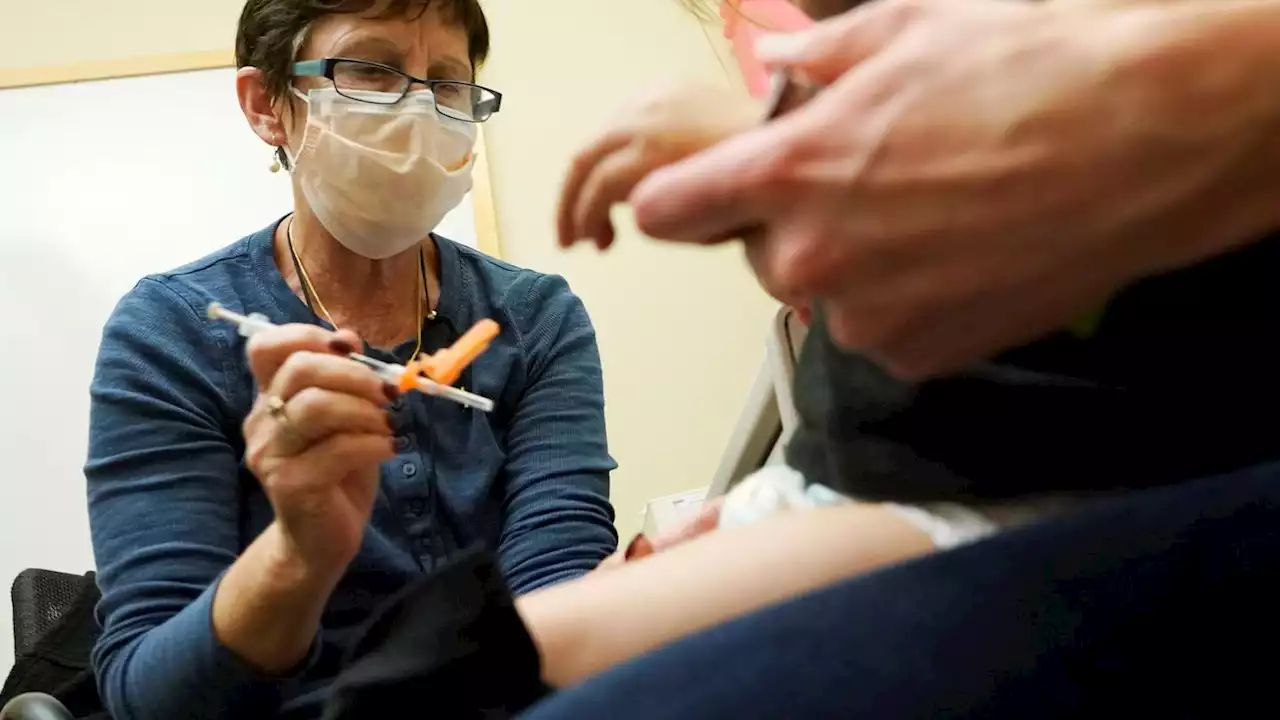 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Leer más »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Leer más »
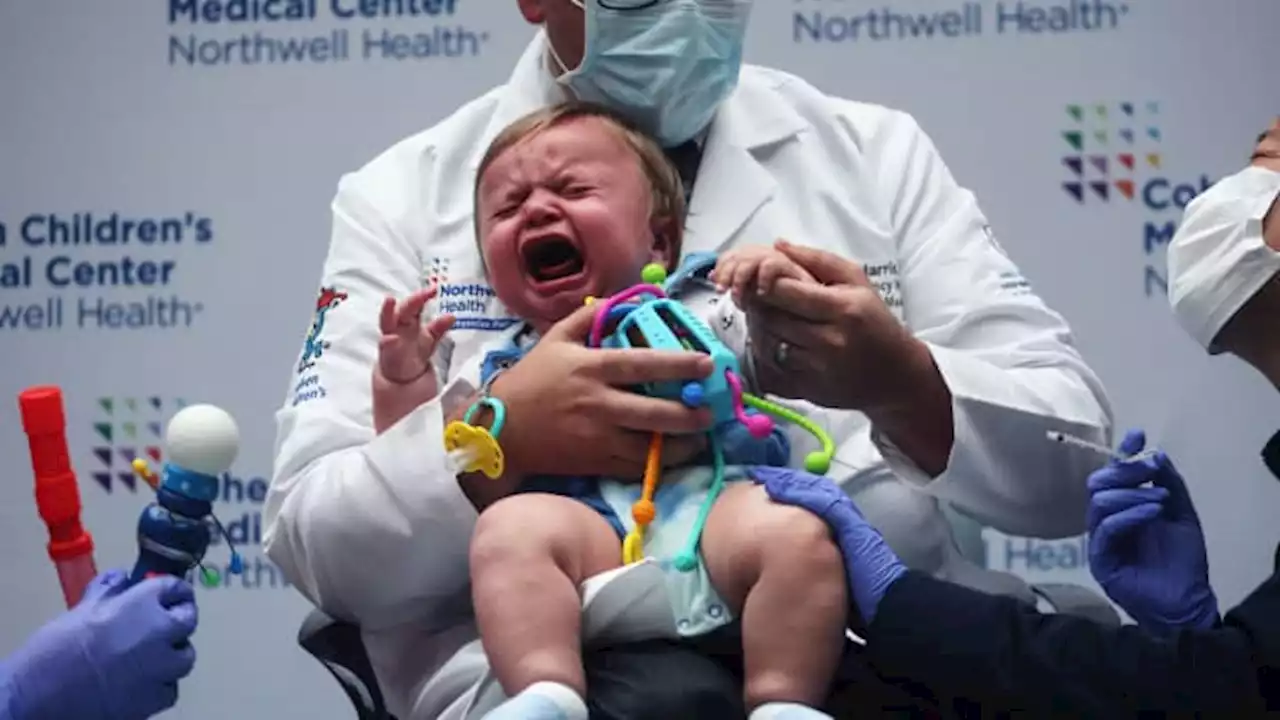 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Leer más »
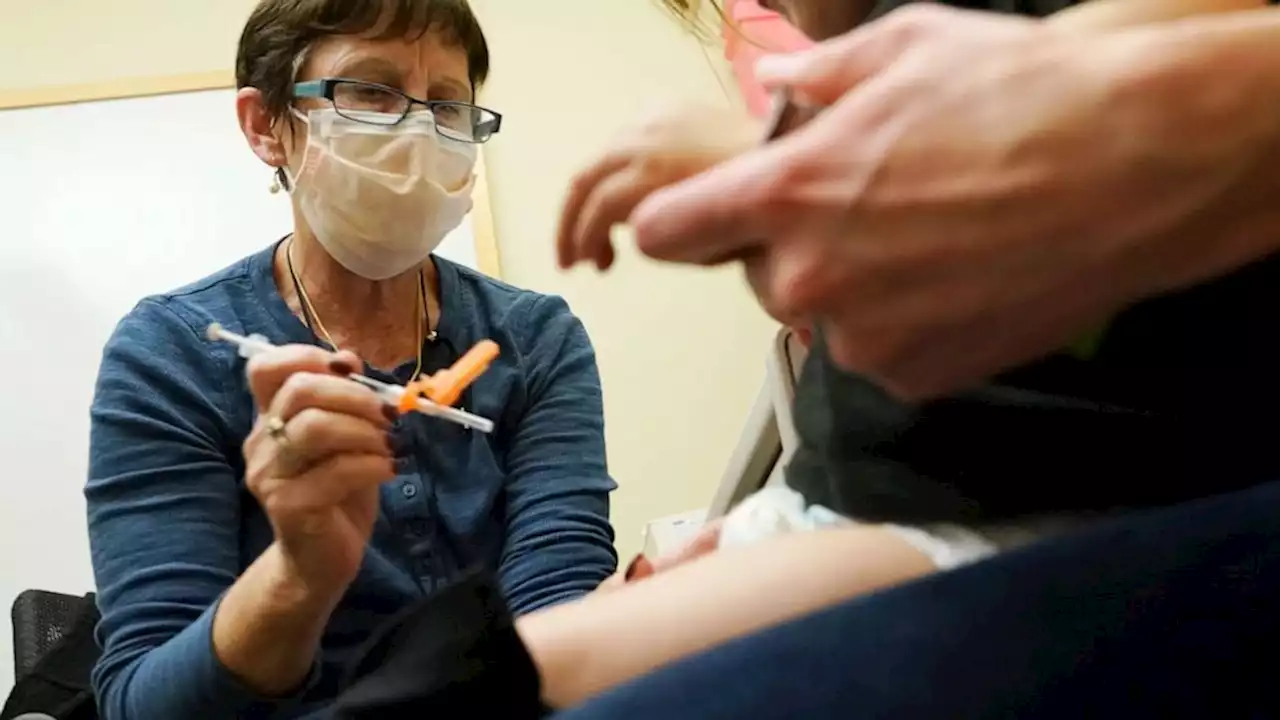 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
Leer más »
