The approval comes just two days after an advisory panel recommended both Moderna and Pfizer vaccines for young children.
Moderna and Pfizer-BioNTech vaccines for children as young as six months, clearing one of the last hurdles for America’s youngest population to get vaccinated more than two years into the pandemic.Key Facts
Pfizer’s vaccine was already available to children and teens ages six to 17, while Moderna’s was not.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
Leer más »
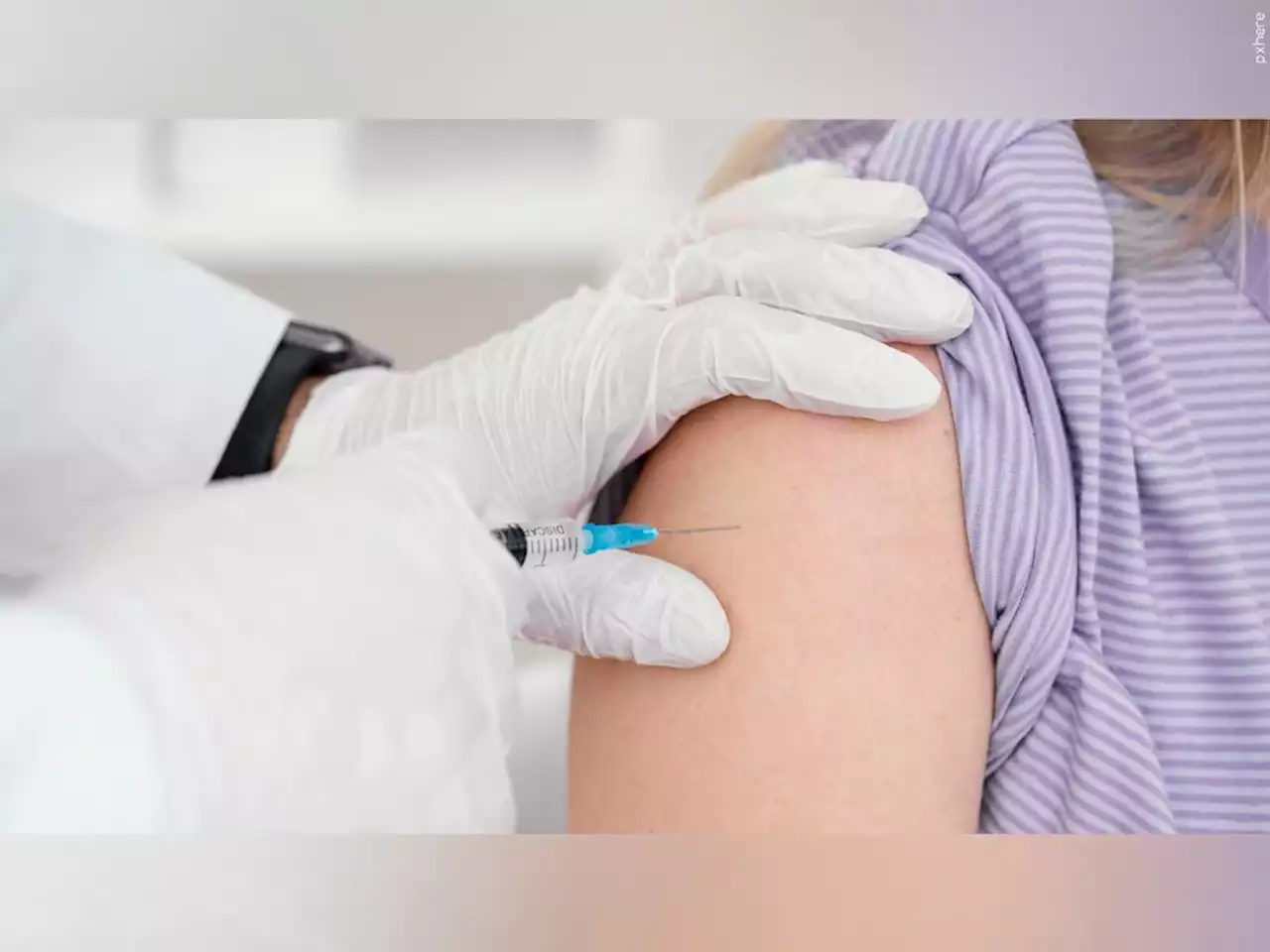 FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
Leer más »
 FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
Leer más »
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Leer más »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Leer más »
 FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
Leer más »
