The FDA approves a new vaccine for COVID-19, new data is released about the risks of myocarditis, and the World Health Organization advises against the use of two different drugs for the treatment of mild to moderate COVID-19.
The U.S. Food and Drug Administrationfor the Novavax COVID-19 Vaccine, Adjuvanted for people 18 and older on Wednesday.
The vaccine, which is administered as a two-dose series, three weeks apart, differs from mRNA vaccines already on the market in that it contains SARS-CoV-2 spike protein and Matrix-M adjuvant. The spike protein in the Novavax vaccine is produced in insect cells; the Matrix M-adjuvant contains saponin extracts from the bark of the Soapbark tree.
The authors reviewed more than 8,000 cases from 46 studies by researchers in Canada and conclude that while cases are rare and usually mild, they occur most frequently among young males shortly after a second dose, and are probably higher after Moderna’s mRNA vaccine than after Pfizer’s vaccine.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
Leer más »
 FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
Leer más »
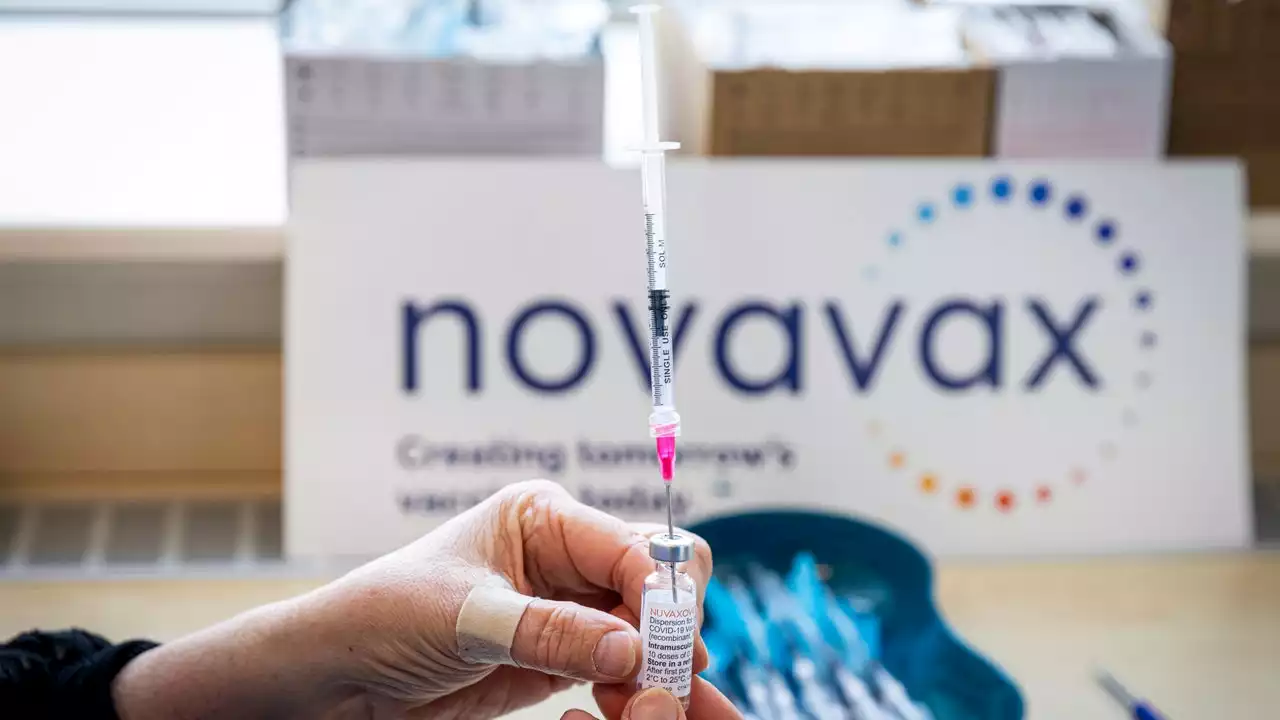 Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Leer más »
 'Classic type of vaccine': FDA approves new COVID-19 shot from Novavax'It is more the classic type of vaccine that we are used to with say, the influenza vaccine,' said Darren Mareiniss, an emergency medicine physician.
'Classic type of vaccine': FDA approves new COVID-19 shot from Novavax'It is more the classic type of vaccine that we are used to with say, the influenza vaccine,' said Darren Mareiniss, an emergency medicine physician.
Leer más »
 Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Leer más »
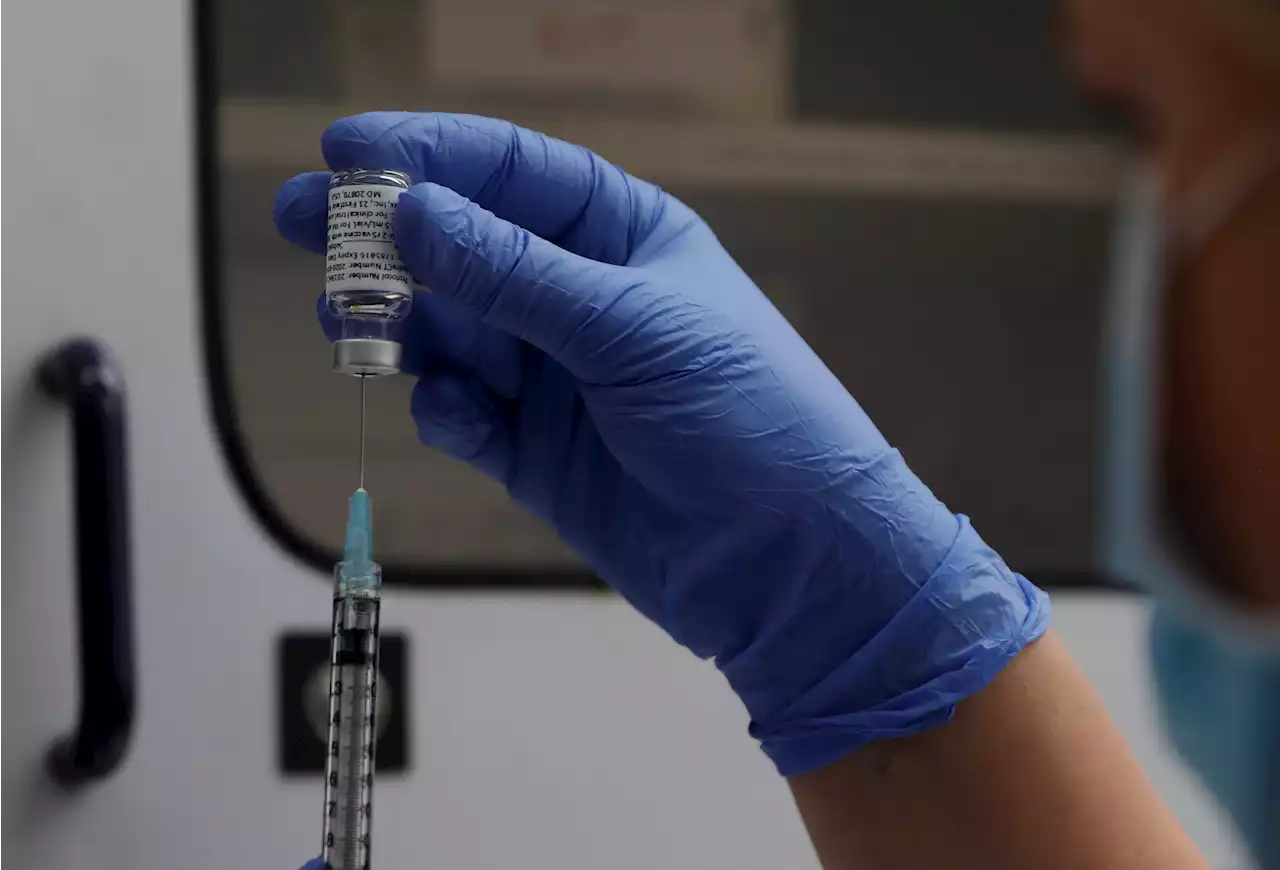 Novavax Covid-19 vaccine wins FDA authorizationThe FDA granted emergency use authorization to Novavax’s Covid-19 vaccine. In a trial of more than 26,000 adults, 2 doses of the Novavax Covid vaccine were over 90% effective at preventing symptomatic disease and presented no serious side effects.
Novavax Covid-19 vaccine wins FDA authorizationThe FDA granted emergency use authorization to Novavax’s Covid-19 vaccine. In a trial of more than 26,000 adults, 2 doses of the Novavax Covid vaccine were over 90% effective at preventing symptomatic disease and presented no serious side effects.
Leer más »
