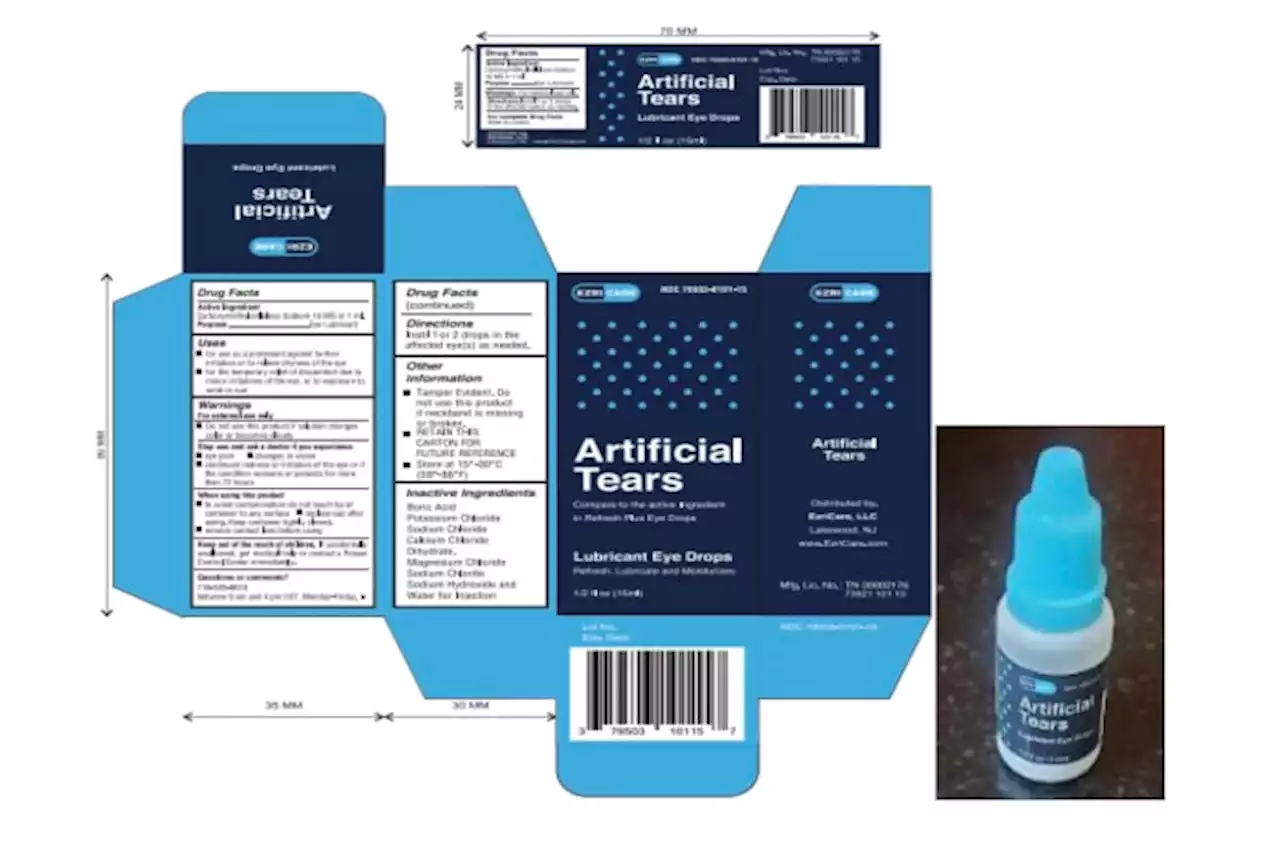
The Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops.
is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
The report has the agency’s preliminary findings and is likely to be followed by a formal report and a warning letter to the company. An FDA spokesman said the inspection indicates that the company’s products “may be in violation of FDA’s requirements.” The FDA is responsible for assuring the safety of foreign products shipped to the U.S., though it has long struggled to keep pace with international pharmaceutical supply chains that increasingly begin in India and China.
Global Pharma has said little publicly about its recent recalls, instead referring questions to the U.S. companies that sold the products.
Argentina Últimas Noticias, Argentina Titulares
Similar News:También puedes leer noticias similares a ésta que hemos recopilado de otras fuentes de noticias.
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Leer más »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Leer más »
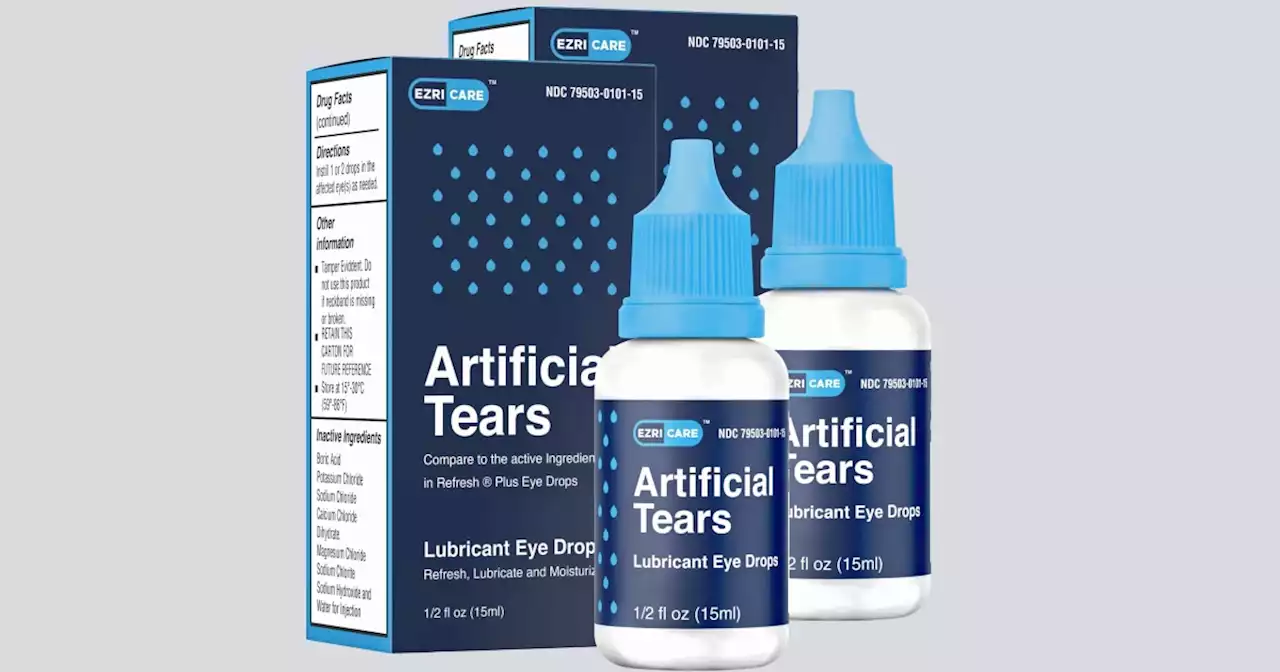 Maker of eyedrops linked to deadly infections couldn’t ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
Maker of eyedrops linked to deadly infections couldn’t ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
Leer más »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors. Food and Drug Administration officials uncovered about a dozen problems with how Global Pharma Healthcare made and tested its eyedrops during an inspection from late February through early March. The FDA released its preliminary inspection report Monday.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors. Food and Drug Administration officials uncovered about a dozen problems with how Global Pharma Healthcare made and tested its eyedrops during an inspection from late February through early March. The FDA released its preliminary inspection report Monday.
Leer más »
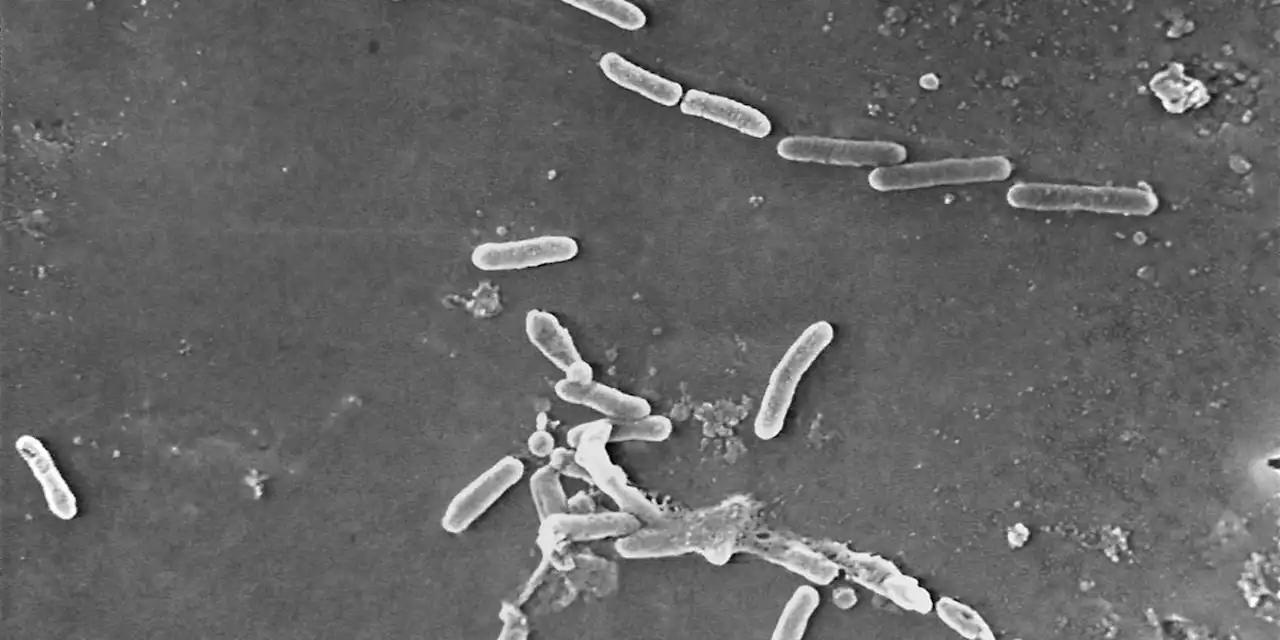 Eyedrops maker couldn’t ensure factory was sterile, FDA saysIn particular, the inspectors found that the plant had used “a deficient manufacturing process” between December 2020 and April 2022 for products that were later shipped to the U.S.
Eyedrops maker couldn’t ensure factory was sterile, FDA saysIn particular, the inspectors found that the plant had used “a deficient manufacturing process” between December 2020 and April 2022 for products that were later shipped to the U.S.
Leer más »